3-chloro-4-methylaniline
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
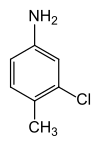
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | 3-chloro-4-methylaniline | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 7 H 8 ClN | ||||||||||||||||||
| Brief description |
solid slowly melting at room temperature |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 141.60 g mol −1 | ||||||||||||||||||
| Physical state |
liquid or solid |
||||||||||||||||||
| density |
1.17 g cm −3 |
||||||||||||||||||
| Melting point |
24 ° C |
||||||||||||||||||
| boiling point |
237-238 ° C |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| Refractive index |
1.584 (20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
3-chloro-4-methylaniline is a chemical compound from the group of aminobenzenes and monochloroanilines . The compound's hydrochloride is also known as Starlicid due to its high toxicity to birds.
Extraction and presentation
3-Chloro-4-methylaniline can be obtained by chlorination of p -nitrotoluene and subsequent catalytic reduction on sulfided palladium / activated carbon contacts.
properties
3-chloro-4-methylaniline is a flammable, hardly ignitable solid that melts slowly at room temperature and is sparingly soluble in water. Yellow-brown liquid at temperatures above the melting point. A contaminated product can be liquid even at room temperature. The decomposition temperature of the compound is above 500 ° C, and the decomposition produces hydrogen chloride, among other things. Two stable and one metastable phases of the compound are known.
use
3-chloro-4-methylaniline is used to produce 2-chloro-4-cyanotoluene by the Sandmeyer reaction with copper (I) cyanide . It can also be used as an intermediate in the production of other chemical compounds such as the herbicide chlortoluron . The hydrochloride of 3-chloro-4-methylaniline has been approved for bird control in the USA since 1967.
Individual evidence
- ↑ a b c d e f g h i j Entry on 3-chloro-4-methylaniline in the GESTIS substance database of the IFA , accessed on November 21, 2018(JavaScript required) .
- ↑ David R. Lide: CRC Handbook of Chemistry and Physics A Ready-reference Book of Chemical and Physical Data . CRC Press, 1995, ISBN 978-0-8493-0595-5 , pp. 114 ( limited preview in Google Book search).
- ↑ a b Data sheet 2-Chloro-4-aminotoluene, 98% from Sigma-Aldrich , accessed on November 21, 2018 ( PDF ).
- ↑ a b EPA: Starlicide (3-chloro-p-toluidine hydrochloride)
- ^ A b Heinz-Gerhard Franck, Jürgen W. Stadelhofer: Industrial Aromatic Chemistry Raw Materials Processes Products . Springer-Verlag, 2013, ISBN 978-3-662-07875-4 , pp. 492 ( limited preview in Google Book search).
- ↑ SK Roy, B. Amitha, J. Uchil: Study of polymorphism and torsional motions in 3-chloro-4-methylaniline using Cl NQR (nuclear quadrupole resonance). In: Canadian Journal of Physics. 70, 1992, p. 119, doi : 10.1139 / p92-015 .

