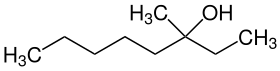
3-methyl-3-octanol
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| Simplified structural formula without stereochemistry | ||||||||||||||||
| General | ||||||||||||||||
| Surname | 3-methyl-3-octanol | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 9 H 20 O | |||||||||||||||
| Brief description |
colorless liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 144.25 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.822 g cm −3 (25 ° C) |
|||||||||||||||
| boiling point |
127 ° C |
|||||||||||||||
| Refractive index |
1.433 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
3-Methyl-3-octanol is a chemical compound from the group of alkanols .
Occurrence
3-Methyl-3-octanol was found in extracts of the fungus Antrodia camphorata . The compound also occurs as a metabolite in the biodegradation of polyethylene and polypropylene .
Extraction and presentation
3-Methyl-3-octanol can be obtained by reacting ethylmagnesium bromide with 2-heptanone . Alternatively, methylmagnesium bromide can be reacted with 3-octanone , or n-pentylmagnesium bromide with butanone . This reaction produces a 1: 1 mixture ( racemate ) of ( R ) -3-methyl-3-octanol and ( S ) -3-methyl-3-octanol, which is also ( RS ) -3-methyl-3- is called octanol.
properties
3-methyl-3-octanol is a colorless liquid.
Individual evidence
- ↑ a b c d e f g h i j data sheet 3-methyl-3-octanol, 99% from Sigma-Aldrich , accessed on January 15, 2019 ( PDF ).
- ↑ Hua Liu, Wei Jia et al. a .: GC-MS and GC-olfactometry analysis of aroma compounds extracted from culture fluids of Antrodia camphorata. In: World Journal of Microbiology and Biotechnology. 24, 2008, p. 1599, doi : 10.1007 / s11274-007-9614-1 .
- ↑ Arutchelvi, J Sudhakar, M Arkatkar, Ambika Doble, Mukesh Bhaduri, Sumit Uppara, Parasu Veera, Biodegradation of polyethylene and polypropylene , IJBT Vol.07 (1), January 2008, accessed January 15, 2019.
- ↑ Allan F. Sowinski, George M. Whitesides: SN2 displacements and reductive coupling of ketones with olefins in N, N-diethylacetamide and N-ethylpyrrolidone. In: The Journal of Organic Chemistry. 44, 1979, p. 2369, doi : 10.1021 / jo01328a008 .
