2-heptanone
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
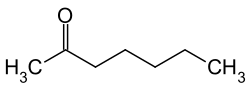
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 2-heptanone | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 7 H 14 O | |||||||||||||||
| Brief description |
colorless liquid with a fruity odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 114.19 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.82 g cm −3 |
|||||||||||||||
| Melting point |
−35 ° C |
|||||||||||||||
| boiling point |
151 ° C |
|||||||||||||||
| Vapor pressure |
|
|||||||||||||||
| solubility |
poorly soluble in water (4.3 g l −1 at 20 ° C) |
|||||||||||||||
| Refractive index |
1.4007 (20 ° C, 589 nm) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| MAK |
Switzerland: 50 ml m −3 or 235 mg m −3 |
|||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
2-Heptanone is a chemical compound from the group of ketones .
Occurrence


2-heptanone occurs naturally as a component of mold odor (e.g. also blue cheese) and blackberries. It is an alarm pheromone in rats.
Extraction and presentation
2-Heptanone can be obtained by reductive condensation of acetone with butyraldehyde in one or two steps, by a ketone decomposition of ethyl butyl acetoacetate or by hydration of 1-heptyne and 2-heptyne .
properties
2-Heptanone is a slightly volatile, colorless liquid with a fruity odor (also spicy like cinnamon) that is sparingly soluble in water.
use
2-Heptanone is used as a high boiler in coating materials. By reaction with ethylmagnesium bromide can be 3-methyl-3-octanol can be obtained.
safety instructions
The vapors of 2-heptanone can - like almost all other liquid organic substances - form an explosive mixture with air ( flash point 39.5 ° C, ignition temperature 305 ° C, lower explosion limit 1.11 vol.%, Upper explosion limit 7.9 vol .-%) form.
Individual evidence
- ↑ a b c d e f g h i j k l m n o p q Entry on 2-heptanone in the GESTIS substance database of the IFA , accessed on October 23, 2018(JavaScript required) .
- ↑ Data sheet 2-heptanone (PDF) from Merck , accessed on December 19, 2012.
- ↑ Entry on Heptan-2-one in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on August 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 110-43-0 or 2-heptanone ), accessed on November 2, 2015.
- ↑ Robert Ebermann, Ibrahim Elmadfa: Textbook food chemistry and nutrition . Springer DE, 2011, ISBN 3-7091-0210-3 , pp. 424 ( limited preview in Google Book search).
- ↑ Wolfgang Legrum: Fragrances, between stench and fragrance: occurrence, properties and ... Springer DE, 2011, ISBN 3-8348-1245-5 , p. 13.67 ( limited preview in Google Book Search).
- ^ Entry on 2-heptanone in the Hazardous Substances Data Bank , accessed December 19, 2012.
- ^ Sabine Krist, Gerhard Buchbauer, Carina Klausberger: Lexicon of vegetable fats and oils . Springer, 2008, ISBN 3-211-75606-X , p. 239 ( limited preview in Google Book search).
- ↑ Wolfgang Mücke, Christa Lemmen: Scent and smell: Effects and health significance of odorous substances . Hüthig Jehle Rehm, 2010, ISBN 3-609-16436-0 , p. 74 ( limited preview in Google Book search).
- ↑ Allan F. Sowinski, George M. Whitesides: SN2 displacements and reductive coupling of ketones with olefins in N, N-diethylacetamide and N-ethylpyrrolidone. In: The Journal of Organic Chemistry. 44, 1979, p. 2369, doi : 10.1021 / jo01328a008 .

