4- tert- butylbenzaldehyde
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
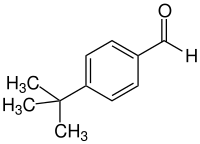
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 4-tert-butylbenzaldehyde | |||||||||||||||
| other names |
p - tert -butylbenzaldehyde |
|||||||||||||||
| Molecular formula | C 11 H 14 O | |||||||||||||||
| Brief description |
colorless liquid with a terpene-like, camphor-like, spicy, fruity odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 162.23 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.973 g cm −3 (20 ° C) |
|||||||||||||||
| Melting point |
<−20 ° C |
|||||||||||||||
| boiling point |
244-246 ° C |
|||||||||||||||
| Vapor pressure |
3–4 Pa (20 ° C) |
|||||||||||||||
| solubility |
very heavy in water (133 mg l −1 at 20 ° C) |
|||||||||||||||
| Refractive index |
1.5270 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
4- tert -Butylbenzaldehyde is a chemical compound from the group of aldehydes .
Extraction and presentation
4- tert -Butylbenzaldehyde can be obtained by oxidizing 4- tert -butyltoluene with hydrogen peroxide in glacial acetic acid , catalyzed by bromide ions in combination with cobalt (II) acetate or cerium (III) acetate .
properties
4- tert -Butylbenzaldehyde is a flammable, hardly inflammable, light- and air-sensitive, colorless liquid with a terpene-like, camphor-like, spicy, fruity odor that is very sparingly soluble in water.
use
4- tert -Butylbenzaldehyde is an important intermediate for the synthesis of drugs, colorants, aromas and fragrances. It is used to manufacture penicillin derivatives with an isoxazolyl partial structure.
Individual evidence
- ↑ a b c d e f g h i Entry on 4-tert-butylbenzaldehyde in the GESTIS substance database of the IFA , accessed on January 9, 2019(JavaScript required) .
- ↑ a b c data sheet 4-tert-butylbenzaldehyde, 97% from Sigma-Aldrich , accessed on January 27, 2019 ( PDF ).
- ^ CRC Handbook of Chemistry and Physics, 95th Edition . CRC Press, 2014, ISBN 978-1-4822-0868-9 .
- ^ Leon G. A. van de Water, Arati Kaza, James K. Beattie, Anthony F. Masters, Thomas Maschmeyer: Partial Oxidation of 4- tert -Butyltoluene Catalyzed by Homogeneous Cobalt and Cerium Acetate Catalysts in the Br - / H 2 O 2 / Acetic Acid System: Insights into Selectivity and Mechanism . In: Chemistry - A European Journal . tape 13 , no. 28 , 2007, pp. 8037-8044 , doi : 10.1002 / chem.200700371 .
- ↑ Data sheet 4-tert-butylbenzaldehyde, 97% from AlfaAesar, accessed on August 24, 2017 ( PDF )(JavaScript required) .

