6: 2-fluorotelomersulfonic acid
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
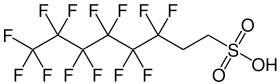
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 6: 2-fluorotelomersulfonic acid | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 8 H 5 F 13 O 3 S | |||||||||||||||
| Brief description |
white to light brown solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 428.17 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.953 g cm −3 (20 ° C) |
|||||||||||||||
| Melting point |
87 ° C |
|||||||||||||||
| pK s value |
1.31 |
|||||||||||||||
| solubility |
658 g l −1 in water (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
6: 2- fluorotelomersulfonic acid ( 6: 2-FTS ) is a chemical compound that belongs to the group of per- and polyfluorinated alkyl compounds (PFAS). Due to its structural similarity to PFOS , it is also called H 4 PFOS .
Extraction and presentation
6: 2 FTS is made by telomerization . It also arises from some perfluorocarboxybetaines after they have been split.
properties
The octanol-water partition coefficient (log K OW ) is 3.47-3.98.
use
6: 2-FTS is used as a substitute for perfluorooctanesulfonic acid (PFOS) or its salts in extinguishing foams . It is also used in hard and Dekorativverchromungsverfahren in the electroplating used as a replacement for the banned PFOS. 6: 2-AGV was initially only pre-registered under REACH , but was then registered in the EEA in 2019 for the tonnage range of 1–10 tonnes per year .
toxicity
The EC 50 for Eisenia fetida is 253 mg / kg (reproduction test).
literature
- N. Wang, J. Liu, RC Buck, SH Korzeniowski, BW Wolstenholme, PW Folsom, LM Sulecki: 6: 2 fluorotelomer sulfonate aerobic biotransformation in activated sludge of waste water treatment plants. In: Chemosphere . Volume 82, Number 6, February 2011, pp. 853-858, doi: 10.1016 / j.chemosphere.2010.11.003 , PMID 21112609 .
Individual evidence
- ↑ a b c d e f Entry on 3,3,4,4,5,5,6,6,7,7,8,8,8-tridecafluorooctanesulfonic acid in the GESTIS substance database of the IFA , accessed on December 18, 2019 (JavaScript required)
- ↑ a b c laenderfinanzierungsprogramm.de: 01_Anhang_A_Grundlagen PFC work aid ( memento from September 23, 2016 in the Internet Archive ), accessed on September 23, 2016.
- ↑ PFT and Analytics ( Memento from September 22, 2016 in the Internet Archive ). LANUV , accessed September 23, 2016.
- ↑ a b The Danish Environmental Protection Agency: Short chain Polyfluoroalkyl Substances (PFAS) , 2015, ISBN 978-87-93352-15-5 , accessed on September 23, 2016.
- ↑ Xiaoling Yang, Jun Huang, Kunlun Zhang, Gang Yu, Shubo Deng, Bin Wang: Stability of 6: 2 fluorotelomer sulfonate in advanced oxidation processes: degradation kinetics and pathway. In: Environmental Science and Pollution Research . 21, 2014, pp. 4634-4642, doi: 10.1007 / s11356-013-2389-z .
- ↑ H. Hauser, L. Füglister, T. Scheffelmaier: Use of fluorosurfactants in the electroplating industry , 2020.
- ↑ InfoCard for 3,3,4,4,5,5,6,6,7,7,8,8,8-tridecafluorooctanesulphonic acid of the European Chemicals Agency (ECHA), accessed on November 18, 2019.


