Acetaldehyde diethyl acetal
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
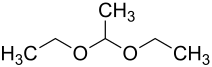
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Acetaldehyde diethyl acetal | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 6 H 14 O 2 | |||||||||||||||
| Brief description |
colorless liquid with a fruity odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 118.18 g · mol -1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.83 g cm −3 |
|||||||||||||||
| Melting point |
−100 ° C |
|||||||||||||||
| boiling point |
102 ° C |
|||||||||||||||
| solubility |
soluble in water (46 g l −1 at 25 ° C) |
|||||||||||||||
| Refractive index |
1.3834 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Acetaldehyde diethyl acetal (also simply acetal ) is a chemical compound with the empirical formula C 6 H 14 O 2 . The colorless, pleasantly smelling liquid is not very soluble in water , but easily soluble in organic solvents. While like all acetals it is sensitive to acids , it remains stable to alkalis . Other names are 1,1-diethoxyethane and diethylacetal.
synthesis
Acetaldehyde diethyl acetal is obtained from acetaldehyde and ethanol by introducing the acetaldehyde into an ethanolic solution of anhydrous calcium chloride . After the mixture has been left to stand for one day at room temperature , the acetaldehyde diethyl acetal formed in the upper phase is separated off.
properties
- Molar heat of combustion = -3870 kJ / mol
use
Acetaldehyde diethyl acetal is primarily a solvent , but it can also be used preparatively as a protected acetaldehyde or as a fuel additive to adjust the octane number .
safety instructions
Acetaldehyde diethyl acetal is highly flammable and has a narcotic effect .
Individual evidence
- ↑ a b c d e data sheet acetaldehyde diethylacetal (PDF) from Merck , accessed on January 19, 2011.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-164.
- ↑ a b Entry on 1,1-diethoxyethane in the GESTIS substance database of the IFA , accessed on February 14, 2017(JavaScript required) .
- ↑ Entry on 1,1-diethoxyethane in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .

