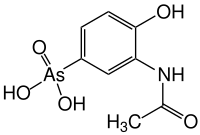
Acetarsol
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||
| General | ||||||||||
| Surname | Acetarsol | |||||||||
| other names |
|
|||||||||
| Molecular formula | C 8 H 10 AsNO 5 | |||||||||
| Brief description |
white solid |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| Drug information | ||||||||||
| ATC code | ||||||||||
| properties | ||||||||||
| Molar mass | 275.09 g mol −1 | |||||||||
| Physical state |
firmly |
|||||||||
| Melting point |
225-227 ° C |
|||||||||
| solubility |
|
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| Toxicological data | ||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Acetarsol is a chemical compound from the group of organic arsenic compounds .
history
Acetarsol was already used by Paul Ehrlich in 1911 as an intermediate for the production of arsphenamine- analogue. Ernest Fourneau arranged the production under the name Stovarsol by Poulenc Freres . It turned out to be a potent remedy for syphilis.
Extraction and presentation
Acetarsol can be obtained from 4-chloroaniline . By reaction thereof with sodium nitrite , Natriumorthoarsenit it is converted to 4-chloro-3-nitro-phenylarsonic acid, which with sodium thiosulfate or sodium hydroxide reacts to form 3-amino-4-hydroxy-phenylarsonic acid, which in turn with acetic anhydride is converted to Acetarsol.
properties
Acetarsol is a white odorless powder with a slightly sour taste that is practically insoluble in cold water and ethanol. The dimethylamine and sodium salts are also white odorless powders, but they are soluble in water.
use
Acetarsol is used to treat amoebiasis and trichomoniasis . The dimethylamine and sodium salts are also used.
safety instructions
Hallucinations and disturbed perceptions may occur when the connection is established. If swallowed, stomach irritation, nausea, vomiting and diarrhea can occur.
Individual evidence
- ↑ a b c d e f g h Data sheet Acetarsone, ≥99% from Sigma-Aldrich , accessed on December 26, 2015 ( PDF ).
- ↑ a b c d F. v. Bruchhausen, Siegfried Ebel, AW Frahm, E. Hackenthal: Hager's handbook of pharmaceutical practice substances AD . Springer-Verlag, 2013, ISBN 978-3-642-57995-0 , pp. 22 ( limited preview in Google Book search).
- ↑ Walter Sneader: Drug Discovery A History . John Wiley & Sons, 2005, ISBN 978-0-471-89979-2 , pp. 55 ( limited preview in Google Book search).
- ↑ a b P. H. List, L. Hörhammer: Chemicals and Drugs (Am - Ch) . Springer-Verlag, 2013, ISBN 978-3-642-80562-2 , p. 252 ( limited preview in Google Book search).