Acetyl iodide
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
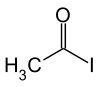
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Acetyl iodide | |||||||||||||||
| other names |
Acetyl iodide |
|||||||||||||||
| Molecular formula | C 2 H 3 IO | |||||||||||||||
| Brief description |
red to dark red liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 169.95 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
2.0674 g cm −3 |
|||||||||||||||
| Melting point |
13 ° C |
|||||||||||||||
| boiling point |
108 ° C |
|||||||||||||||
| Vapor pressure |
67 mbar (36 ° C) |
|||||||||||||||
| solubility |
Reacts with water |
|||||||||||||||
| Refractive index |
1.5491 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
| Thermodynamic properties | ||||||||||||||||
| ΔH f 0 |
−163.5 kJ / mol |
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Acetyl iodide is a chemical compound from the group of carboxylic acid halides and organic iodine compounds .
Extraction and presentation
Acetyl iodide can be obtained by reacting acetaldehyde with iodine .
properties
Acetyl iodide is a red to dark red liquid that is sensitive to light and moisture.
use
Acetyl iodide is formed as an intermediate product in the synthesis of acetic acid during the classic Monsanto process or the Hoechst-Celanese process through rhodium- catalyzed formation from methyl iodide and carbon monoxide . It reacts with water to form hydrogen iodide and acetic acid.
Individual evidence
- ↑ a b c d Entry on Acetyl Iodide at TCI Europe, accessed on January 21, 2012.
- ↑ a b c d e Entry for CAS no. 507-02-8 in the GESTIS substance database of the IFA , accessed on January 21, 2012(JavaScript required) .
- ↑ a b Dirk Steinborn: Fundamentals of organometallic complex catalysis . Vieweg + Teubner, 2009, ISBN 978-3-8348-0581-2 ( page 100 in the Google book search).
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-6.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Standard Thermodynamic Properties of Chemical Substances, pp. 5-22.
- ↑ Entry on Acetyl iodide . In: P. J. Linstrom, W. G. Mallard (Eds.): NIST Chemistry WebBook, NIST Standard Reference Database Number 69 . National Institute of Standards and Technology , Gaithersburg MD .



