Esculetin
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
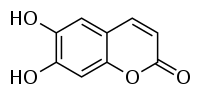
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Esculetin | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 9 H 6 O 4 | |||||||||||||||
| Brief description |
light yellow solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 178.14 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
271-273 ° C |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Esculetin is a coumarin derivative. In nature it occurs, among other things, in ash bark ( Cortex Fraxini ), which is used in traditional Chinese medicine . The pharmacological effect of ash bark is based, among other things, on the presence of esculetin and its glycoside esculin .
effect
Esculetin is an antioxidant and can therefore protect DNA from damage caused by oxidative stress . This effect could e.g. B. be shown in experiments with cell cultures. Esculetin was able to intercept reactive oxygen species (ROS), which can be formed by UV light . This observation suggests a preventive effect of esculetin against skin cancer . Natural remedies containing esculetin are also used in folk medicine to treat liver damage. A preventive effect of esculetin against liver damage caused by paracetamol could actually be proven in experiments with mice. The liver-protecting effect is probably based on the reaction of esculetin with the free radicals formed by the liver enzyme cytochrome P450 . Further possible effects against u. a. Diabetes , obesity or leukemia could be determined in animal and cell culture experiments.
synthesis
Esculetin can be produced synthetically from p -benzoquinone . This is first reacted in a 1,4-addition with acetic anhydride to form hydroxyhydroquinone triacetate . The latter reacts with malic acid to form esculetin. Both of these partial reactions require sulfuric acid as a catalyst .
Another possibility for synthesis consists in the reaction of hydroxyhydroquinone with ethyl propiolate . Zinc chloride acts as a catalyst in this reaction .
Individual evidence
- ↑ a b c d e data sheet 6,7-dihydroxycoumarin, 98% from Sigma-Aldrich , accessed on January 20, 2017 ( PDF ).
- ↑ a b Mao, G., Zhang, S., Song, H., Ding, S., Zhu, P., Wang, X., Liang, C .: Synthesis, biological activities and therapeutic properties of esculetin and its derivatives . In: JOCPR . tape 7 , no. 4 , 2015, p. 122-130 ( PDF ).
- ↑ Kaneko, T., Tahara, S., Takaba, F .: Suppression of Lipid Hydroperoxide-Induced Oxidative Damage to Cellular DNA by Esculetin . In: Biol Pharm Bull. Volume 26 , no. 6 , 2003, p. 840-844 , doi : 10.1248 / bpb.26.840 ( PDF ).
- ↑ Gilani, AH, Janbaz, KH, Shah, BH: Esculetin prevents liver damage induced by paracetmaol and CCl 4 . In: Pharmacol Res. Volume 37 , no. 1 , 1998, p. 31-35 , doi : 10.1006 / phrs.1997.0262 .
- ↑ Lin, W.-L., Wang, C.-J., Yu-Ying, T., Liu, C.-L., Hwang, J.-M., Tseng, T.-H .: Inhibitory effect of esculetin on oxidative damage induced by t-butyl hydroperoxide in rat liver . In: Arch. Toxicol. tape 74 , 2000, pp. 467-472 , doi : 10.1007 / s002040000148 .
- ↑ Cao, WQ; Xue, JF; Shi, CM; Thing, CF; Zhu, XG; Liu, F .; Zhou, XJ: 6,7-Dihydroxycoumarins . In: Fine Chem. Intermed. tape 43 , no. 3 , 2013, p. 39-41 (Chinese).
- ↑ Yang, XJ; Gao, HH: Study on the synthesis of esculetin under microwave irradiation . In: Appl. Chem. Ind. Volume 40 , no. 4 , 2011, p. 627-629 (Chinese, English abstract ).
