Imine
Imines are derivatives of aldehydes or ketones . Imines are compounds in which the oxygen atom of the aldehyde or ketone has been formally replaced by a nitrogen atom. The nitrogen atom also carries a hydrogen atom or an organic radical R 3 ; in the latter case, one speaks of an azomethine or a Schiff base , named after Hugo Schiff (1834–1915), the German-Italian chemist and discoverer of this class of substances. The structural design is therefore R 1 R 2 C = NR 3 . R 1 , R 2 and R 3 can be different or the same radicals, including hydrogen atoms. Aldimines (R 1 or / and R 2 = H) are N analogs of aldehydes and ketimines (R 1 and R 2 = organyl radical , for example alkyl or aryl radical ) N analogs of ketones .
Heterocyclic amines such as piperidine ( pentamethyleneimine ) were also referred to as imines (especially in the older literature) . Today, however, this designation is inadmissible because real imines have the typical imine double bonds .
properties
Imines are less basic than corresponding amines, since the lone pair of electrons of the sp 2 - hybridized nitrogen is less available for the attachment of a proton . With suitable substitution, imines are subject to imine-enamine tautomerism .
Manufacturing
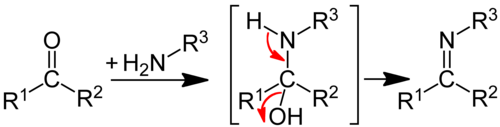
Imines are formed from primary amines with aldehydes or ketones; Half- aminals , the analogs of the half-acetals, are formed as an intermediate .
The reaction occurs through a nucleophilic addition between a carbonyl compound and a primary amine . An unstable hemiaminal is formed via a zwitterion, which now has a positively charged nitrogen and a negatively charged oxygen atom. In three further steps, a proton (protonated) is first attached to the oxygen, and the oxygen receives a positive charge. After the removal of water from the carbon and the last hydrogen atom from the nitrogen, an azomethine is formed. The formation of enamines also proceeds analogously . The Asinger reaction produces heterocyclic imines (e.g. 3- thiazolines ) with a C = N double bond as part of a five-membered ring system.
use
In some multi-component reactions (e.g. Strecker synthesis , Ugi reaction ) for the production of amino acids or amino acid derivatives, imines occur as intermediate products or are used as starting materials. Heterobimetal catalysts derived from BINOL are used in the enantioselective addition of phosphorus nucleophiles to imines.
Web links
Individual evidence
- ^ Brockhaus ABC Chemie , VEB FA Brockhaus Verlag Leipzig 1965, p. 668.
- ↑ IUPAC definition of imines
- ^ Ivan Ernest: Binding, Structure and Reaction Mechanisms in Organic Chemistry , Springer-Verlag, 1972, pp. 194–195, ISBN 3-211-81060-9 .
- ↑ Jürgen Martens , Heribert Offermanns and Paul Scherberich: A simple synthesis of racemic cysteine , Angewandte Chemie 93 (1981) 680 doi : 10.1002 / anie.19810930808 ; Angewandte Chemie International Edition English 20 (1981) 668. doi : 10.1002 / anie.198106681 .
- ^ H. Gröger , Y. Saida, H. Sasai, K. Yamaguchi, J. Martens and M. Shibasaki : A New and Highly Efficient Asymmetric Route to Cyclic alpha-Amino Phosphonates: The first Catalytic Enantioselective Hydrophosphonylation of Cyclic Imines Catalyzed by Chiral Heterobimetallic Lanthanoid Complexes. In: J. Am. Chem. Soc. 120 ( 1998 ) 3089-3103, doi : 10.1021 / ja973872i .
- ^ I. Schlemminger, Y. Saida, H. Gröger, W. Maison, N. Durot, H. Sasai, M. Shibasaki, J. Martens: Concept of Rigidity: How to Make Enantioselective Hydrophosphonylation of Cyclic Imines Catalyzed by Chiral Heterobimetallic Lanthanoid Complexes almost perfect. In: J. Org. Chem. 65 ( 2000 ) 4818-4825, doi : 10.1021 / jo991882r .