Allylescaline
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||
| General | |||||||||||||
| Surname | Allylescaline | ||||||||||||
| other names |
|
||||||||||||
| Molecular formula | C 13 H 19 NO 3 | ||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| Drug information | |||||||||||||
| Drug class | |||||||||||||
| properties | |||||||||||||
| Molar mass | 237.29 g · mol -1 | ||||||||||||
| Physical state |
firmly |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Allylescaline is a lesser-known psychedelic intoxicant .
chemistry
| Structural similarity of allylescaline and mescaline |
|---|
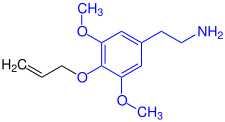
Allylescaline |

Mescaline |
| The analog structural elements are marked in blue . |
The chemical structure is closely related to that of mescaline . Allylescaline belongs to the phenylethylamines group , which also includes the neurotransmitter dopamine , the hormone adrenaline and the synthetic amphetamine . Allylescaline was first synthesized in 1972 by the Czech chemist Otakar Leminger. The compound was later synthesized by Alexander Shulgin and described in detail in his book " Pihkal: A Chemical Love Story ".
Effects
According to Alexander Shulgin's description, allylescaline should lead to an elevated mood with psychedelic components and deepened awareness. The effect should therefore at least tend to be comparable to other hallucinogens such as mescaline or LSD . The dosage range is indicated by Shulgin with about 20-35 mg and the duration of the effect with about 8-12 hours. In addition, very little data exist on the pharmacological properties of allylescaline.
Legal status
In Germany, allylescaline is listed as 4-allyloxy-3,5-dimethoxy-phenethylazane in Appendix I to the Narcotics Act. Despite the ending -azan , it is not a member of the azane group , but an amine .
literature
- SJ Chapman, AA Avanes: PeakAL: Protons I Have Known and Loved - Fifty Shades of Gray-Market Spectra. In: Blotter. Aug 1, 2015. doi: 10.16889 / isomerdesign-1
Web links
- isomerdesign.com: Allylescaline (English)
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Otakar Leminger: The Chemistry of Alkoxylated Phenethylamines - Part 2 . In: Chemický průmysl. 22, 1972, p. 553.
- ↑ Leminger, Otakar, 1875-1929. In: Bibliography dějin Českých zemí. (Bibliography of Czech Chemistry)
- ↑ PIHKAL - Phenethylamines I Knew and Loved . In: Erowid . (English)
- ↑ # 2 AL . In: Erowid . (English)