Aluminum sodium dioxide
| Crystal structure | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
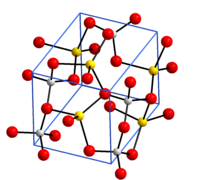
| Crystal structure of aluminum sodium dioxide __ Al 3+ __ Na + __ O 2− |
||||||||||||||||
| General | ||||||||||||||||
| Surname | Aluminum sodium dioxide | |||||||||||||||
| other names |
|
|||||||||||||||
| Ratio formula | NaAlO 2 | |||||||||||||||
| Brief description |
white odorless solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 81.97 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.52 g cm −3 |
|||||||||||||||
| Melting point |
1650-1850 ° C |
|||||||||||||||
| solubility |
easily in water (~ 500 g l −1 at 20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Aluminum sodium dioxide or sodium metaluminate is a chemical compound with the ratio formula NaAlO 2 from the group of metal oxides , sodium compounds and aluminum compounds , more precisely the sodium aluminates . It crystallizes in an orthorhombic structure with space group Pna 2 1 (space group # 33.) Consisting eckenverküpften AlO 4 - tetrahedra is. The Na + ions are also tetrahedrally surrounded by O 2− ions.
Extraction and presentation
Sodium aluminates generally arise when sodium hydroxide solution acts on aluminum salts , with the sodium hydroxide solution being used in excess and a sodium aluminate solution being formed.
use
Aluminum sodium dioxide is used as a flocculant in sewage treatment , as a construction chemical ( quick hardener for concrete ) and in the manufacture of paints and soaps . The sodium aluminate solution is of far greater technical importance .
Individual evidence
- ↑ a b c d e Entry on aluminum sodium dioxide in the GESTIS substance database of the IFA , accessed on July 23, 2016(JavaScript required) .
- ↑ Safety data sheet for sodium aluminate Bernd Kraft. Retrieved January 1, 2018.
- ↑ J. A. Kaduk, S.-Y. Pei: The crystal structure of hydrated sodium aluminate, Na Al O 2 · 5/4 H 2 O, and its dehydration product . In: Journal of Solid State Chemistry , 1995 , 115 , pp. 126-139.
- ↑ Patent DE60001390 : Fast-setting cement containing lime and aluminates. Registered on March 2, 2000 , published on December 11, 2003 , applicant: Italcementi SpA, inventors: Umberto Costa, Lorenzo Barcella.


