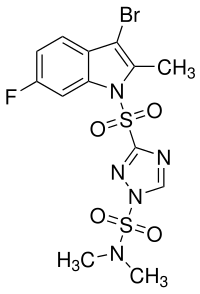
Amisulbrom
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Amisulbrom | |||||||||||||||
| other names |
3- (3-bromo-6-fluoro-2-methylindol-1-ylsulfonyl) - N , N- dimethyl-1 H -1,2,4-triazol-1-sulfonamide |
|||||||||||||||
| Molecular formula | C 13 H 13 BrFN 5 O 4 S 2 | |||||||||||||||
| Brief description |
colorless and odorless powder |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 466.31 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.85 g cm −3 |
|||||||||||||||
| Melting point |
128.6-130.0 ° C |
|||||||||||||||
| boiling point |
242 ° C (decomposition) |
|||||||||||||||
| solubility |
practically insoluble in water (0.11 mg l −1 at 20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Amisulbrom is a chemical compound from the group of sulfonic acid amides and triazoles . The compound was introduced as a fungicide by Nissan Chemical in 2008 .
Extraction and presentation
A.
Two-step synthesis of 3-bromo-6-fluoro-2-methyl-1 H -indole (A) from a Pd catalyst.
B.
Formation of 1- ( N , N -dimethylsulfamoyl) -1 H -1,2,4-triazole-3-sulfonyl chloride (B)
Amisulbrom
A and B react in the presence of sodium hydroxide , tetrabutylammonium and br-toluene to synthesize the end product amisulbromine .
use
Amisulbrom is one of the QiI fungicides that inhibit cytochrome c reductase at the Qi site. It is used against Phytophthora infestans on potatoes (late blight and tuber rot) and tomatoes ( late blight and brown rot ), against Plasmopara viticola on wine (downy mildew of grapevines) and against powdery mildew on vegetables, fruit and ornamentals.
Admission
Amisulbrom was added to the list of approved active ingredients by the EU Commission for use as a fungicide in 2014. Today it is approved as an active ingredient in plant protection products in many EU countries, including Germany and Austria, as well as Switzerland.
Individual evidence
- ↑ a b c d e f Entry on Amisulbrom. In: Römpp Online . Georg Thieme Verlag, accessed on March 4, 2014.
- ↑ a b Entry on Amisulbrom in the Pesticide Properties DataBase (PPDB) of the University of Hertfordshire , accessed on March 4, 2014.
- ↑ a b Amisulbrom data sheet from Sigma-Aldrich , accessed on May 22, 2017 ( PDF ).
- ↑ entry on amisulbrom (ISO); 3- (3-bromo-6-fluoro-2-methylindol-1-ylsulfonyl) -N, N-dimethyl-1H-1,2,4-triazole-1-sulfonamide in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA) , accessed on December 29, 2019. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Wolfgang Krämer, Ulrich Schirmer, Peter Jeschke, Matthias Witschel: Modern Crop Protection Compounds: Herbicides, Volume 1 . Wiley-VCH, 2011, ISBN 978-3-527-32965-6 , pp. 620 ( limited preview in Google Book search).
- ↑ Implementing Regulation (EU) No. 193/2014 of February 27, 2014
- ↑ General Directorate Health and Food Safety of the European Commission: Entry on Amisulbrom in the EU pesticide database ; Entry in the national registers of plant protection products in Switzerland , Austria and Germany ; accessed on March 20, 2016.




