Ammonium chlorate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 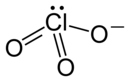
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Ammonium chlorate | |||||||||||||||
| Molecular formula | NH 4 ClO 3 | |||||||||||||||
| Brief description |
colorless solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 101.49 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.91 g cm −3 |
|||||||||||||||
| Melting point |
100 ° C (decomposition) |
|||||||||||||||
| solubility |
easily soluble in water |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Ammonium chlorate is a chemical compound from the group of ammonium compounds and chlorates .
Extraction and presentation
Ammonium chlorate can be obtained by reacting potassium chlorate with ammonium sulfate .
It can also be done by reacting a chloric acid solution with the stoichiometrically necessary amount of ammonia or ammonium carbonate
or by reaction of barium chlorate with ammonium sulfate.
properties
Ammonium chlorate is an inconsistent colorless solid that is very easily soluble in water and is in the form of small needle-shaped crystals. Care must be taken when handling the compound, as the substance sometimes explodes for no apparent reason, but certainly at temperatures above 100 ° C. Spread openly in a thin layer, the substance is safe to handle. It has a trigonal crystal structure with the space group R 3 m (space group no. 160) .
Web links
Individual evidence
- ^ A b c d e Jean d'Ans, Ellen Lax, Roger Blachnik: Pocket book for chemists and physicists . Springer DE, 1998, ISBN 3-642-58842-5 , pp. 588 ( limited preview in Google Book search).
- ↑ a b c d e Georg Brauer , with the collaboration of Marianne Baudler u. a. (Ed.): Handbook of Preparative Inorganic Chemistry . 3rd, revised edition. tape I . Ferdinand Enke, Stuttgart 1975, ISBN 3-432-02328-6 , pp. 324 .
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.



