Arsenic (III) iodide
| Crystal structure | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| __ As 3+ __ I - | ||||||||||||||||
| General | ||||||||||||||||
| Surname | Arsenic (III) iodide | |||||||||||||||
| other names |
|
|||||||||||||||
| Ratio formula | AsI 3 | |||||||||||||||
| Brief description |
red solid with a pungent odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 455.64 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
4.38 g cm −3 |
|||||||||||||||
| Melting point |
141.8 ° C |
|||||||||||||||
| boiling point |
403 ° C |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Thermodynamic properties | ||||||||||||||||
| ΔH f 0 |
−58.2 kJ / mol |
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Arsenic (III) iodide is an inorganic chemical compound of arsenic from the group of iodides .
Extraction and presentation
Arsenic (III) iodide can be obtained by reacting arsenic (III) chloride with potassium iodide or from the elements.
It can also be obtained from arsenic (III) oxide by reacting with hydrochloric acid and a potassium iodide solution.
properties
Arsenic (III) iodide is a red-orange solid with a pungent odor that slowly decomposes in water.
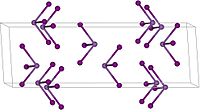
In air it gradually decomposes to iodine and arsenic (III) oxide . It has a trigonal crystal structure with the space group R 3 (space group no.148) . From 110 ° C, the compound is a high-temperature modification with a crystal structure with the space group P 3 2 12 (No. 153) .
use
Arsenic (III) iodide was previously used to treat dermatitis in cats and as Donovan's solution to treat skin inflammation and other conditions.
Individual evidence
- ↑ a b c d e f g h i Entry for CAS no. 7784-45-4 in the GESTIS substance database of the IFA , accessed on April 24, 2016(JavaScript required) .
- ^ Franz von Bruchhausen, Siegfried Ebel, Eberhard Hackenthal: Hager's Handbook of Pharmaceutical Practice: Substances AK . Springer DE, 1999, ISBN 978-3-642-58387-2 , pp. 107 ( limited preview in Google Book search).
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Standard Thermodynamic Properties of Chemical Substances, pp. 5-5.
- ↑ a b Entry on arsenic (III) iodide in the Hazardous Substances Data Bank , accessed on August 5, 2013.
- ↑ Georg Brauer (Ed.), With the collaboration of Marianne Baudler a . a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume I, Ferdinand Enke, Stuttgart 1975, ISBN 3-432-02328-6 , p. 575.
- ^ Jean D'Ans, Ellen Lax: Pocket book for chemists and physicists . Springer DE, 1997, ISBN 3-540-60035-3 , pp. 306 ( limited preview in Google Book search).





