Biochanin A
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
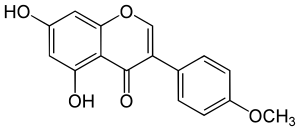
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Biochanin A | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 16 H 12 O 5 | ||||||||||||||||||
| Brief description |
light yellow needles |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 284.26 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
212 ° C |
||||||||||||||||||
| solubility |
25 g l −1 (in acetone ) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Biochanin A belongs to the isoflavone class of flavonoids . It is also classified as phytoestrogens because it is a herbal, non- steroidal compound that has - weak - estrogen-like biological activity.
Occurrence
Biochanin A was found in certain legume species, mainly in red clover (also called meadow clover ).
biosynthesis
Starting from the flavone apigenin , its (4-hydroxyphenyl) group is first rearranged from the 2-position to the 3-position, with a hydroxyl group being introduced at the 2-position under the influence of NADPH / H + and oxygen . The result is 2-hydroxy-2,3-dihydrogenistein, which enzymatically eliminates water and thus becomes genistein . Methylation of the hydroxy group in the 4-position of the phenyl radical with S- adenosylmethionine finally yields the biochanin A.
Individual evidence
- ↑ Entry on isoflavones. In: Römpp Online . Georg Thieme Verlag, accessed on June 20, 2014.
- ↑ a b c Datasheet Biochanin A from Sigma-Aldrich , accessed on May 25, 2011 ( PDF ).
- ↑ L. Bialesová, J. Brtko, V. Lenko, D. Macejová: Nuclear receptors - target molecules for isoflavones in cancer chemoprevention. In: General physiology and biophysics. Volume 32, Number 4, December 2013, pp. 467-478, doi : 10.4149 / gpb_2013064 . PMID 24067281 .