Bis (salicylidene) ethylenediamine
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
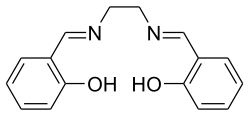
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Bis (salicylidene) ethylenediamine | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 16 H 16 N 2 O 2 | |||||||||||||||
| Brief description |
pale yellow, glowy solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 268.32 g · mol -1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
127-128 ° C |
|||||||||||||||
| solubility |
almost insoluble in water |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Bis (salicylid) ethylenediamine (H 2 -Salen) is a chemical compound which, after deprotonation of the two OH functions, is used as a chelating ligand ( Salen ) in coordination chemistry and homogeneous catalysis .
Manufacturing
Bis (salicylidene) ethylenediamine can be prepared by reacting salicylaldehyde and ethylenediamine in ethanol .
properties
It is a light yellow, micaceous solid that dissolves in polar organic solvents .
See also
literature
- P. Pfeiffer, E. Breith, E. Lübbe, T. Tsumaki: Tricyclic orthocondensed secondary valence rings . In: Justus Liebig's Annals of Chemistry . tape 503 , no. 1 , 1933, pp. 84-130 , doi : 10.1002 / jlac.19335030106 .
- Harvey Diehl, Clifford C. Hach, John C. Bailar: Bis (N, N'-Disalicylalethylenediamine) -μ-Aquodicobalt (II) . In: Ludwig F. Audrieth (Ed.): Inorganic Syntheses . tape 3 . John Wiley & Sons, Inc., 1950, ISBN 978-0-470-13234-0 , pp. 196-201 , doi : 10.1002 / 9780470132340.ch53 .
Individual evidence
- ↑ a b c d e f g Data sheet N, N′-Bis (salicylidene) ethylenediamine, 98% from Sigma-Aldrich , accessed on March 20, 2015 ( PDF ).



