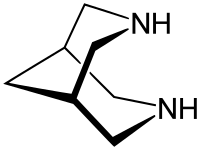
Bispidine
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||
| General | ||||||||||
| Surname | Bispidine | |||||||||
| other names |
3,7-diazabicyclo [3.3.1] nonane |
|||||||||
| Molecular formula | C 7 H 14 N 2 | |||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 126.20 g mol −1 | |||||||||
| Physical state |
firmly |
|||||||||
| Melting point |
158-161 ° C |
|||||||||
| boiling point |
190-195 ° C (9 torr) |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Bispidine is a bicyclic, heterocyclic compound whose systematic name is 3,7-diazabicyclo [3.3.1] nonane.
Occurrence
Bispidine forms the basic body of some alkaloids such as B. Sparteine and Cytisine .
presentation
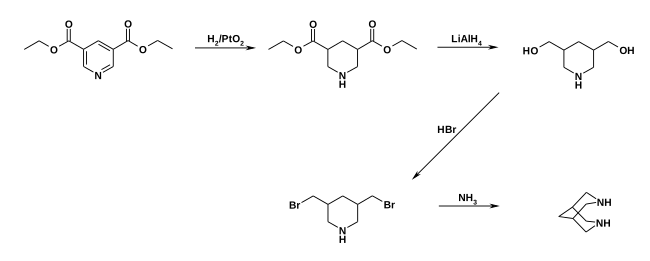
Bispidine can be synthesized by a Raney nickel- catalyzed hydrogenation of pyridine-3,5-carbonitrile. A multi-stage synthesis starts from pyridine-3,5-dicarboxyethyl ester, which is first hydrogenated over platinum oxide to give piperidine-3,5-dicarboxyethyl ester. A reduction by means of lithium aluminum hydride then yields 3,5-bis (hydroxymethyl) piperidine. By nucleophilic substitution, the dialcohol is first converted into the dibromide and then into the diamide, which cyclizes to the target compound.
A more recent synthesis is based on allylamine and ethyl acrylate , where initially the N, N'-diallylbispidinone is formed in a double Mannich reaction via 1-allylpiperidin-4-one. A subsequent Wolff-Kishner reduction and deallylation with ethyl chloroformate gives the bispidine.
Bispidine derivatives can be prepared, for example, by selective Michael additions .
properties
Bispidine is a white crystalline solid that begins to sublime at 135 ° C. A melting point of 158–161 ° C can be observed in the closed tube.
The reaction with formaldehyde gives the diazaadamantane.
use
Bispidine derivatives are used in chemistry as chelating ligands for transition metals .
Individual evidence
- ↑ a b c Y. Miyahara, K. Goto, T. Inazu: Convenient Synthesis of 3,7-Diazabicyclo [3.3.1] nonane (bispidine) in Synthesis 2001, 364-366, doi : 10.1055 / s-2001-11427 .
- ↑ H. Stetter, R. Merten: About connections with urotropin structure, IX. For knowledge of bispidine in Chem. Ber. 90 (1957) 868-875, doi : 10.1002 / cber.19570900605
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ D. Hoppe, T. Hense: Enantioselective synthesis with lithium / (-) - sparteine-carbanion pairs in Angew. Chem. 109 (1997) 2376-2410, doi : 10.1002 / anie . 19971092105 .
- ↑ D. Stead, P. O'Brien, A. J. Sanderson: Concise Synthesis of (±) -Cytisine via Lithiation of N-Boc-bispidine in Org. Lett. 7 (2005) 4459-4462, doi : 10.1021 / ol0516869 .
- ↑ F. Bohlmann, N. Ottawa, R. Keller: Structure of the Tetrahydroquinolizons and the "Bispidins" contributions to the synthesis of the cytisine in Liebigs Ann. Chem. 587 (1954) 162-176, doi : 10.1002 / jlac.19545870210 .
- ↑ a b F. Galinovsky, H. Langer: Synthesis of 1,3-diaza-adamantans and bispidins in monthly journals for chemistry 86 (1955) 449-453, doi : 10.1007 / BF00903631 .
- ↑ M. Breuning, M. Steiner: Convenient Multigram Synthesis of (R) -Homopipecolic Acid Methyl Ester in Synthesis 2006, 1386-1389, doi : 10.1055 / s-2006-926419 .
- ↑ M. Breuning, D. Hein: First asymmetric synthesis of a C2-symmetric 2-endo, 6-endo-disubstituted bispidine in Tetrahedron Asym. 18 (2007) 1410-1418, doi : 10.1016 / j.tetasy.2007.06.010 .
- ↑ University of Würzburg: Chiral Bispidines and 9-Oxabispidines ( Memento of the original from June 24, 2013 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ↑ F. Galinovsky, F. Sparatore, H. Langer: A new synthesis of Tetrahydro-deoxy-cytisins. To the knowledge of bispidine in monthly magazine for chemistry 87 (1956) 100-105, doi : 10.1007 / BF00903593
- ↑ P. Comba, M. Maurer, P. Vadivelu: Oxidation of Cyclohexane by High-Valent Iron Bispidine Complexes: Tetradentate versus Pentadentate Ligands in Inorg. Chem. 48 (2009) 10389-10396, doi : 10.1021 / ic901702s .
