Sparteine
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
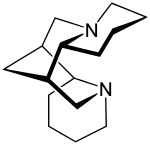
|
|||||||||||||||||||
| Structural formula of (-) - Sparteine | |||||||||||||||||||
| General | |||||||||||||||||||
| Non-proprietary name | Sparteine | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 15 H 26 N 2 | ||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| Drug information | |||||||||||||||||||
| ATC code | |||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 234.38 g · mol -1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.02 g cm −3 |
||||||||||||||||||
| Melting point |
30.5 ° C |
||||||||||||||||||
| boiling point |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Sparteine [more precisely (-) - Sparteine, L -sparteine; also lupinidine ] is a natural product from the group of lupine alkaloids . It has a stimulating effect on the heart , circulation and intestines and increases diuresis . Sparteine is toxic to humans and can lead to death from circulatory collapse.
The enantiomer (+) - sparteine, also called pachycarpine , occurs only rarely. That is why “Spartein” - unless otherwise stated - always means the (-) - Spartein.
Occurrence
(-) - Sparteine is found, for example, in broom and celandine . In the seeds of the yellow lupine ( Lupinus luteus ) the alkaloid content consists of 30–50% sparteine.
Problems with the commercial availability of (-) - sparteine have been reported for some years, causing the prices to rise significantly. In connection with this, research on the chiral chemical, which used to be easily and cheaply accessible, has also declined significantly.
(+) - Sparteine (pachycarpine) is much rarer, it is very toxic and occurs in various legumes , e.g. B. in the US lupine species Lupinus pusillus .
Historical
Sparteine was discovered by John Stenhouse , who obtained it by acid extraction and distillation from the broom (Latin also Spartium scoparium ) and who described the discovery in 1850/1851. He described it as a volatile organic base and a colorless oil, significantly denser than water, with a faint, aniline- like odor and a bitter taste. Sparteine, in the form of sparteine sulfate, has been used to treat heart disease since 1873. Since 1939 it was used as a means of labor. In the 1980s it was still considered a reliable and safe effective antiarrhythmic of first choice for emergency medicine, and a reliable contraceptive.
The first total syntheses of Sparteine were announced in 1948. The first syntheses required a resolution. The first asymmetric total synthesis of the (+) - sparteine isomer uses a variant of the Beckmann rearrangement and was published in 2002.
properties
Sparteine is hardly soluble in cold water. The solution is clearly alkaline. It dissolves easily in ethanol , diethyl ether , dichloromethane and chloroform . Sparteine is insoluble in gasoline ( petroleum ether or ligroin ) and benzene . It is unstable in light and air and turns yellowish to brown in color. Sparteine smells similar to aniline and tastes intensely bitter.
The orally lethal dose for mice is 220 mg / kg body weight.
Structurally, the sparteine is characterized by a bridged ring system and four chiral carbon atoms: In the center there is a symmetrical heterocycle that is bridged by a methylene group . Another ring (A and D) is fused onto two sides , so that a tetracyclic is formed. In addition to the enantiomer (+) - sparteine, there are also α-isosparteine (C-11 epimer) and β ‑ isosparteine (C-6 epimer) as epimers to sparteine.
| Naturally occurring isomers | Structural formula | Stereochemical arrangement | Position of the rings A / BC / D |
| (-) - Sparteine (lupinidine) | 6 R , 7 S , 9 S , 11 S |
cis-trans ( exo / endo ) |
|
| (+) - Sparteine (pachycarpine) | 6 S , 7 R , 9 R , 11 R | ||
| (-) - α-isosparteine ( genistein ) | 6 R , 7 S , 9 S , 11 R |
cis-cis ( endo / endo ) |
|
| (-) - β ‑ isosparteine ( Pusillin , Spartalupine) | 6 R , 7 R , 9 R , 11 R |
trans-trans ( exo / exo ) |
Sparteine biosynthesis
The tetracyclic sparteine is built up from the amino acid L- lysine during biosynthesis . Initially, three molecules of cadaverine are formed from three molecules of L- lysine . In the course of further reactions, four molecules of pyruvate take up four of the amine groups; four molecules of alanine are produced . The biosynthesis takes place in chloroplasts ; then the alkaloids are transported to the other parts of the plant, especially to the ripening fruits.
use
Sparteine sulfate has been used as an antiarrhythmic , i.e. H. used to treat cardiac arrhythmias . It could be given for severe symptomatic tachycardias when the doctor judged them to be life-threatening. Class I antiarrhythmics are rarely used. Sparteine was also used in obstetrics in the past. However, in some cases the labor was unusually violent, and in some cases the fetus died. This drug side effect has been explained by hereditary polymorphisms . Sparteine is used in biomedical research as a model substance for studying this polymorphism, which affects the oxidation of substances by cytochrome P450 .
Similar to bispidine, sparteine can serve as a bidentate ligand with its two nitrogen atoms . In contrast to bispidine, sparteine is chiral . (-) - Sparteine is used as an effective tool in enantioselective synthesis .
Pachycarpine is used medicinally in the CIS for the treatment of hypertensive crises, spasms of the peripheral vessels and myopathies .
Sparteine sulfate
Sparteine sulfate (Sparteinum sulfuricum, sulfuric acid Sparteine) forms colorless, needle-shaped crystals. In contrast to sparteine itself, they are easily soluble in water and a neutral solution is formed. In addition, sparteine sulfate is soluble in alcohol but not in chloroform.
Individual evidence
- ↑ a b c d e data sheet (-) - Sparteine at Sigma-Aldrich , accessed on April 23, 2011 ( PDF ).
- ↑ a b David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-462.
- ↑ Michael Wink, Methods for the Detection of Lupine Alkaloids ( Memento from February 5, 2016 in the Internet Archive )
- ↑ Stephen K. Ritter: Where has all the sparteine gone? | April 24, 2017 Issue - Vol. 95 Issue 17 | Chemical & Engineering News. Retrieved July 22, 2019 .
- ↑ a b H. J. Roth: Symmetrical alkaloids * . In: Deutsche Apothekerzeitung . tape 18, 2010 ( deutsche-apotheker-zeitung.de ).
- ^ Léo Marion, Stuart W. Fenton: The Papilionaceous Alkaloids. VI. Lupinus pusillus, Pursh. In: ACS (Ed.): The Journal of Organic Chemistry (JOC) . tape 13 , no. 5 , September 1948, p. 780-781 , doi : 10.1021 / jo01163a025 .
- ^ A b John Stenhouse : On the Action of Nitric Acid on Various Vegetables, with a More Particular Examination of Spartium scoparium, Linn., Or Common Broom . In: The Royal Society (Ed.): Philosophical Transactions R. Soc. Lond. tape 141 . London January 1, 1851, p. 413-431 , doi : 10.1098 / rstl.1851.0020 , JSTOR : 108406 .
- ↑ John Stenhouse: On the Action of Nitric Acid on Various Vegetables, with a More Particular Examination of Spartium scoparium, Linn., Or Common Broom. Abstract. In: Royal Society (Ed.): Abstracts of the Papers Communicated to the Royal Society of London . 1850-1854. Volume 6 . Taylor and Francis, London 1854, pp. 3-6 , JSTOR : 111121 ( online at Internet Archive [accessed February 2, 2016]).
- ^ A b John Stenhouse: On the effect of nitric acid on various vegetables, together with a closer study of Spartium Scoparium. Linn. In: Friedrich Wöhler, Justus Liebig, Hermann Kopp (Hrsg.): Annalen der Chemie und Pharmacie . tape 78 , no. 1 . CF Winter, Heidelberg 1851, p. 1–30 ( online at the Internet Archive [accessed February 2, 2016]).
- ↑ a b Peter W. Thies: Spartium and Spartein . From broom to antiarrhythmic. In: Pharmacy in our time . tape 15 , no. 6 . VCH, Weinheim November 1986, p. 172-176 , doi : 10.1002 / pauz.19860150604 .
- ↑ Burritt W. Newton, Ralph C. Benson, Colin C. McCorriston: Sparteine sulfate: a potent, capricious oxytocic . In: American Journal of Obstetrics and Gynecology . tape 94 , no. 2 , January 15, 1966, p. 234-241 .
- ^ Nelson J. Leonard, Roger E. Beyler: The Total Synthesis of Sparteine . In: ACS (Ed.): Journal of the American Chemical Society . JACS. tape 70 , no. 6 June 1948, p. 2298–2299 , doi : 10.1021 / ja01186a522 .
- ↑ Nelson J. Leonard, Roger E. Beyler: The Total Synthesis of Sparteine and an Isosparteine by Reductive Cyclization . In: ACS (Ed.): Journal of the American Chemical Society . JACS. tape 72 , no. 3 , March 1950, p. 1316-1323 , doi : 10.1021 / ja01159a067 .
- ↑ Raissa M. Trend: Sparteine - A lupine alkaloid ( Memento from April 4, 2015 in the Internet Archive ) (English, with an overview of the syntheses)
- ^ Brenton T. Smith, John A. Wendt, Jeffrey Aubé: First Asymmetric Total Synthesis of (+) - Sparteine . Letter. In: ACS (Ed.): Organic Letters . tape 4 , no. 15 , July 2002, p. 2577-2579 , doi : 10.1021 / ol026230v .
- ↑ Biochemisches Handlexikon: Volume 5: Alkaloids, Animal Poisons, Products of Internal Secretion, Antigens, Ferments, edited by H. Altenburg et al., Pages 114–117 in the Google book search
- ↑ Hans Günther Boit: Results de Alkaloid-Chemie up to 1960, with special consideration of the progress since 1950 . Akademie-Verlag, 1961, p. 194 .
- ↑ External identifiers of or database links to (+) - Spartein : CAS number: 492-08-0, EC number: 805-899-0, ECHA InfoCard: 100.232.882 , PubChem : 7014 , ChemSpider : 10254876 , Wikidata : Q66604441 .
- ↑ Gerhard Richter Metabolic Physiology of Plants: Physiology and Biochemistry of Primary and Secondary Metabolism. Georg Thieme Verlag, 1998 in the Google book search.
- ↑ Description of the application and its restrictions at arznei-telegramm.de
- ↑ Clinical pharmacology: drug therapy, edited by N. Rietbrock, AH Staib, D. Loew, page 66, right column in the Google book search
- ↑ Clinical Pharmacology: Medicinal therapy, edited by N. Rietbrock, AH Staib, D. Loew, page 68 in the Google book search
- Jump up ↑ SV Otton, T. Inaba, W. Kalow: Competitive Inhibition Of Sparteine Oxidation In Human Liver By β-Adrenoceptor Antagonists And Other Cardiovascular Drugs . In: Life Sciences . tape 34 , no. 1 , January 1984, pp. 73-80 , doi : 10.1016 / 0024-3205 (84) 90332-1 .
- ↑ Dieter Hoppe , Thomas Hense: Enantioselective synthesis with lithium / ( -) - sparteine-carbanion pairs . In: Angewandte Chemie . tape 109 , no. 21 , February 1, 2006, p. 2376-2410 , doi : 10.1002 / anie.19971092105 .
- ↑ E. Teuscher: Biogenic Medicines. 5th edition. Wissenschaftliche Verlagsgesellschaft, 1997. ISBN 3-8047-1482-X . P. 356.





