Proconvertin
| Factor VIIa | ||
|---|---|---|
|
||
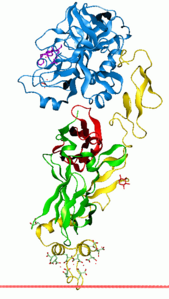
| Thromboplastin (blue) complexed with factor VIIa according to PDB 1DAN | ||
|
Existing structural data: s. UniProt |
||
| Properties of human protein | ||
| Mass / length primary structure | 406 = 152 + 254 amino acids | |
| Secondary to quaternary structure | Heterodimer | |
| Cofactor | Ca 2+ | |
| Isoforms | A, B | |
| Identifier | ||
| Gene names | F7 ; | |
| External IDs | ||
| Drug information | ||
| ATC code |
B02 BD01 B02 BD05 |
|
| DrugBank | DB00036 | |
| Drug class | Antihemorrhagics | |
| Enzyme classification | ||
| EC, category | 3.4.21.21 , serine protease | |
| MEROPS | S01.215 | |
| Substrate | Arg - + - Ile in factor X | |
| Products | Factor Xa | |
| Occurrence | ||
| Homology family | Serine protease | |
| Parent taxon | Euteleostomi | |
| Orthologue | ||
| human | House mouse | |
| Entrez | 2155 | 14068 |
| Ensemble | ENSG00000057593 | ENSMUSG00000031443 |
| UniProt | P08709 | P70375 |
| Refseq (mRNA) | NM_000131 | NM_010172 |
| Refseq (protein) | NP_000122 | NP_034302 |
| Gene locus | Chr 13: 113.11 - 113.12 Mb | Chr 8: 13.03 - 13.04 Mb |
| PubMed search | 2155 |
14068
|
Proconvertin (English: Proconvertin or serum prothrombin conversion accelerator (SPCA); synonyms : stable factor , prothrombinogen and coagulation factor VII ) is an enzyme involved in blood clotting . The synthesis takes place in the liver and is dependent on vitamin K . Proconvertin has a molecular mass of 59 kDa and belongs to the class of β-globulins .
genetics
The gene for proconvertin is on chromosome 13 (13q34).
physiology
The main function of Proconvertin is to activate the coagulation process. If the blood vessels are damaged, the tissue factor present in the tissue (factor III, tissue thrombokinase, tissue thromboplastin) enters the blood and, together with calcium , activates proconvertin. This activated proconvertin (factor VIIa) now activates, together with calcium and phospholipids , factor X (Stuart Prower factor) and factor IX (Christmas factor).
Diseases
Diseases caused by a mutation in proconvertin are rare (1 in 500,000 in the general population) and are inherited as an autosomal recessive trait.
Therapeutic use
Recombinant proconvertin (NovoSeven ® ) was presented as a therapeutic measure for uncontrollable bleeding in hemophilia patients who had formed inhibitors against replacement coagulation factors.
It can be used for uncontrollable bleeding. The hope is that it will only start clotting where there is already thromboplastin. However, studies have shown an increased risk of deep vein thrombosis , pulmonary embolism and myocardial infarction when using recombinant factor VIIa. Another disadvantage of this therapy is its extremely high price.
Individual evidence
- ↑ Harold R. Roberts, Dougald M. Monroe, Gilbert C. White: The use of recombinant factor VIIa in the treatment of bleeding disorders. In: Blood , Vol. 104 (2004), Issue 13, pp. 3858-3864, PMID 15328151 .
- ↑ Kathryn A. O'Connell, Jennifer J. Wood, Robert P. Wise, Jay N. Lozier, M. Miles Braun: Thromboembolic adverse events after use of recombinant human coagulation factor VIIa. In: The Journal of the American Medical Association (JAMA), 295 (2006), 293-298.