Cathelicidine
| Cathelicidine | ||
|---|---|---|
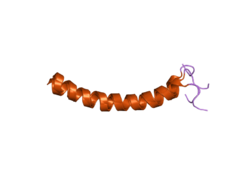
|
||
| 3D structural model of LL-37 | ||
| Mass / length primary structure | 12 - 80 amino acids | |
| Transporter classification | ||
| TCDB | 1.C.33.1.10 | |
| designation | Cathelicidin family ( Porine ) | |
| Occurrence | ||
| Parent taxon | Vertebrates | |
Cathelicidins are antimicrobial peptides that are mainly produced in immune cells of vertebrates and are part of the innate immune response and the apoptosis of the body's own cells. These are transport proteins whose incorporation into the cell wall of Gram-positive bacteria on the one hand and into the cell membrane on the other hand leads to a loss of ions and small molecules. In humans this is CAMP - gene known that for two cathelicidins codes that are cut from a precursor peptide: FALL-39 andLL-37 .
The production of cathelicidin in particular by stimulation of the TLR-9 through, but also TLR-2 and TLR-4 and indirectly vitamin D stimulated.
Occurrence
Cathelicidins are widely used as antimicrobial peptides in the class (biology) of mammals . They are particularly numerous and varied in the representatives of the group of Cetartiodactyla , to which in particular the ungulates belong. Like most other mammals, humans have only a single cathelicidin gene . Outside of the mammalian class, cathelicidins could only be detected sporadically, for example in domestic chickens . Cathelicidin-like peptides could also be found in rainbow trout and hagfish . Due to this distribution, the evolutionary history of the cathelicidins can be estimated to be between 500 and 300 million years ago.
biochemistry
structure
The structurally heterogeneous family of Cathelicidins houses antimicrobial peptides with a length of 12 to 80 amino acid building blocks . The largest group of cathelicidins consists of 23 to 37 amino acids comprising peptides with an α-helix structure. This group includes the human antimicrobial peptides LL-37 and FALL-39. Another group are the peptides consisting of 12 to 18 amino acids with a β-loop structure, which are stabilized via one or two disulfide bridges . These include, for example, the protegrins of pigs. A third group of antimicrobial peptides of the cathelicidins consists of 39 to 80 amino acids, which are predominantly linear peptides with polyproline motifs. Among them are Bac5 and Bac7 of beef and the Prophenine of pig. Bovine indolicidin has a different structure from the other cathelicidins . Indolicidin is a 13 amino acid linear peptide rich in tryptophan . They all consist of a C-terminal cationic domain, which is activated after cleavage from a holoprotein and is responsible for antimicrobial activity.
biosynthesis
The biosynthesis and activation of cathelicidins is a multi-stage process. The human cathelicidin LL-37 is encoded by the CAMP gene on chromosome 3 . Like the cathelicidin genes in other mammals, this gene consists of four exons . The antimicrobial activity is encoded by the hypervariable exon 4, while exons 1 to 3 encode a signal peptide sequence and the subsequent cathelin domain.
Prepropeptides are primarily synthesized and stored in the granules of neutrophil granulocytes or other cells. After these biologically inactive propeptides have been released, they are enzymatically cleaved by elastase or other proteinases into a cathelin and a C-terminal antimicrobial peptide.
See also
literature
- Margherita Zanetti: The Role of Cathelicidins in the Innate Host Defenses of Mammals. In: Current issues in Molecular Biology. Vol. 7, July 2005, ISSN 1467-3037 , pp. 179-196, PMID 16053249 , online (PDF; 345 kB) .
Individual evidence
- ↑ Search result Cathelicidins by taxonomy
- ↑ UniProt P49913
- ↑ Bruno Rivas-Santiago, Rogelio Hernandez-Pando, Claudia Carranza, Esmeralda Juarez, Juan Leon Contreras, Diana Aguilar-Leon, Martha Torres, Eduardo Sada: Expression of cathelicidin LL-37 during Mycobacterium tuberculosis infection in human alveolar macrophages, monocytes, neutrophils , and epithelial cells. In: Infection and Immunity . Vol. 76, No. 3, March 2008, ISSN 0019-9567 , pp. 935-941, PMID 18160480 , doi : 10.1128 / IAI.01218-07 , PMC 2258801 (free full text).
- ↑ Jae-Min Yuk, Dong-Min Shin, Hye-Mi Lee, Chul-Su Yang, Hyo Sun Jin, Kwang-Kyu Kim, Zee-Won Lee, Sang-Hee Lee, Jin-Man Kim, Eun-Kyeong Jos: Vitamin D3 induces autophagy in human monocytes / macrophages via cathelicidin. In: Cell Host & Microbe. Vol. 6, No. 3, September 2009, ISSN 1931-3128 , pp. 231-243, PMID 19748465 , doi : 10.1016 / j.chom.2009.08.004 .
- ↑ a b L. Tomasinsig, M. Zanetti: The cathelicidins - structure, function and evolution. In: Current Protein & Peptide Science. Vol. 6, No. 1, February 2005, ISSN 1389-2037 , pp. 23-34, PMID 15638766 .
- ↑ Thomas Uzzella, Ethan D. Stolzenberg, Ann E. Shinnar, Michael Zasloff: Hagfish intestinal antimicrobial peptides are ancient cathelicidins. In: Peptides . Vol. 24, No. 11, November 2003, pp. 1655-1567, PMID 15019197 , doi : 10.1016 / j.peptides.2003.08.024 .