Cefamandolnafat
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
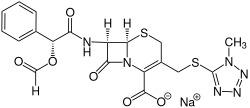
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Cefamandolnafat (INNm) | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 19 H 17 N 6 NaO 6 S 2 | |||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| Mechanism of action | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 512.5 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Cefamandolnafate is the international non-proprietary name (INN) of the sodium salt of the formic acid ester of cefamandol . Since cefamandolnafate is hydrolyzed very quickly in the blood plasma , it is a prodrug of the drug cefamandol.
properties
Cefamandolnafate is a white to almost white, odorless powder. It is easily soluble in water, but only with difficulty in methanol. It occurs as a mixture with cefamandol sodium and sodium carbonate.
synthesis
If cefamandol is reacted with formic acid to form formate and then the sodium salt of 2-ethylhexanoic acid is added in acetone , cefamandolnafate is formed.
storage
The substance must be tightly closed, protected from light and moisture. A safety lock is also necessary for sterile substances. In addition, depending on how it is stored, it can be used within 24 hours to a maximum of 4 days after production.
stability
In solids with a water content of less than 0.1%, hydrolysis does not occur . The rate of hydrolysis increases with rising temperatures (over 50 ° C) and higher water content. At high temperatures and extreme pH values, the molecule splits.
Decomposition products
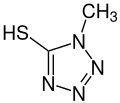
1-methyl-1H- tetrazole -5-thiol
Individual evidence
- ↑ a b c data sheet Cefamandol nafate from Sigma-Aldrich , accessed on April 8, 2020 ( PDF ).
- ↑ a b c d e f Entry on Cefamandol. In: Römpp Online . Georg Thieme Verlag, accessed on April 1, 2020.
- ^ The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals , 14th Edition (Merck & Co., Inc.), Whitehouse Station, NJ, USA, 2006, ISBN 978-0-911910-00-1 , pp. 318.
- ↑ European Pharmacopoeia , Deutscher Apotheker Verlag Stuttgart, 6th edition, 2008, ISBN 978-3-7692-3962-1 , pp. 2000–2002.
- ↑ Axel Kleemann , Jürgen Engel, Bernd Kutscher and Dieter Reichert: Pharmaceutical Substances , Thieme-Verlag Stuttgart, 5th edition (2009) ISBN 978-3-13-558405-8 , pp. 166-167; also online with biannual additions and updates.
- ^ Eugene C. Rickard, Gary G. Cooke: Electrochemical Analysis of the Cephalosporin Cefamandole Nafate . In: Journal of Pharmaceutical Sciences, 1977, Vol. 66, No. 3, pp. 379-384, doi: 10.1002 / jps.2600660317 .