Chain reaction (chemistry)
A chemical chain reaction is a reaction in which a start event triggers a reaction whose intermediate product (often radicals) in whole or in part serves as the starting point for one or more subsequent reactions and which is ended by a termination reaction.
history
It has been known since Max Planck's research that light consists of discrete quanta . The excitation of a single chemical reaction by a light quantum could be explained by this, but not the extreme light sensitivity of some reactions, in which the quantum yield was in the order of magnitude of 10 4 to 10 6 .
Max Bodenstein , who had already completed his habilitation on gas reactions in chemical kinetics in 1899 , had the idea in 1913 that this was due to a chain reaction. This means that when two molecules react, not only can the end product of the reaction arise, but also unstable molecules that can continue the chain.
The Danish chemist Jens Anton Christiansen and the Dutch physicist Hendrik Anthony Kramers pointed out in 1923 that chain reactions can also be triggered by the collision of two molecules. They also investigated the possibility of the chain reaction branching out with the possibility of an explosion.
During investigations into phosphorus combustion in 1926, Juli Borissowitsch Chariton and Z. Valta found that the reaction was surprisingly reduced at an oxygen content above a certain concentration. The continuation of these experiments by Nikolai Nikolajewitsch Semjonow led to the discovery of the explosion limits and a confirmation of the ideas of Kramers and Christiansen.
Semjonow and Hinshelwood also investigated the oxyhydrogen reaction, for which they were awarded the Nobel Prize in Chemistry in 1956 “for their research on the mechanisms of chemical reactions” .
Chain reactions are of great technical and economic importance in free-radical and cationic polymerization. Chlorine radicals are of considerable environmental relevance. As chain starters, they break down around 100,000 ozone molecules in a chain reaction and thus contribute to the breakdown of the ozone layer .
Unbranched chain reaction
If only one radical is generated per radical in radical chain reactions, one speaks of unbranched chain reactions.
An example of this is the chlorine gas reaction , in which hydrogen and chlorine react to form hydrogen chloride according to the following equation:
The reaction takes place on exposure or thermal activation with the participation of hydrogen and chlorine radicals as chain carriers .
The simple kinetic description of the system is achieved using Bodenstein's quasi-stationarity principle . The quasi-stationary principle is based on the assumption that the concentration of the intermediate products formed is constant over time, i.e. the rate of formation is equal to the rate of further reaction.
For the general follow-up reaction
with the reaction rate constants and the following reaction rates result:
Applying Bodenstein's quasi-stationary principle, it is assumed that the concentration of B remains unchanged.
- .
This results in:
and
- .
Chain reactions with polymer products
A chain reaction of great economic importance is the radical polymerization of olefins . A so-called radical starter such as dibenzoyl peroxide is used to start the chain, i.e. a compound that easily breaks down thermally or photolytically into radicals. This attaches to an olefin and thus generates a primary radical to which other olefins attach to form a carbon-carbon bond. The reaction is terminated either by disproportionation (self-inhibition) or the addition of radical scavengers (external inhibition). The average chain length (average degree of polymerization ) can be controlled via the ratio of the initial concentrations of radical initiator and monomer.
Branched chain reactions
If several radicals are generated per radical in radical chain reactions, one speaks of branched chain reactions.
The oxyhydrogen reaction is an example of a branched chain reaction in which hydrogen and oxygen react to form water according to the following equation:
The reaction takes place with the participation of hydrogen, oxygen and hydroxyl radicals as chain carriers .
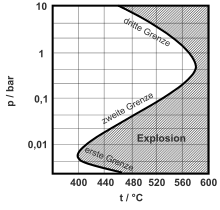
Depending on the pressure, temperature and composition of the mixture, an explosion will occur if the chain branching reactions are more likely than the chain termination reactions. If the pressure is too low or if the oxygen concentration is too high, the termination reactions are more likely than the chain branches and the system is in an area below the lower explosion limit . At higher pressure and in the correct mixing ratio , the lower explosion limit is exceeded and the mixture explodes. Even higher pressure or a further increase in the hydrogen concentration means that the chain termination reactions predominate again, for example through recombination, and the overall reaction speed is slowed down.
See also
literature
- WJ Moore, DO Hummel, G. Trafara, K. Holland-Moritz: Physical chemistry , 1236 pages, Gruyter publishing house (1986), ISBN 3-11-010979-4
- J. Bülle, A. Hüttermann: The basic knowledge of organic chemistry , Verlag Wiley-VCH, ISBN 3-527-30847-4 ( limited preview in the Google book search), p. 33.
Individual evidence
- ↑ Entry on chain reaction . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.C00960 .
- ^ Handbook of waste water and recycling technology for the metalworking industry , by Ludwig Hartinger.
- ↑ M. Bodenstein: On the kinetics of the chlorine explosion gas. Z. phys. Chem. 85 (1913) 329.
- ↑ J. A. Christiansen, HA Kramers: Z. Phys. Chem. 104 (1923) 451.
- ↑ Master of modern physics: the scientific contributions of HA Kramers , by D. ter Haar.
- ↑ Nikolai Nikolajewitsch Semjonow: Some problems relating to chain reactions and to the theory of combustion (PDF; 133 kB), Nobel Prize lecture 1956.
- ^ Cyril Norman Hinshelwood: Chemical kinetics in the past few decades (PDF; 54 kB), Nobel Prize lecture 1956.
- ↑ Chlorine in the World: or Good Bad? , by David P. Gilkey and Holly A. Williams.
- ^ Polystyrene by Hermann Gausepohl, D. Braun, Roland Gellert.










![{\ frac {d [A]} {dt}} = - k_ {1} [A]](https://wikimedia.org/api/rest_v1/media/math/render/svg/9aab0300de29397d5427fbce43de34407edaca68)
![{\ frac {d [B]} {dt}} = k_ {1} [A] -k_ {2} [B]](https://wikimedia.org/api/rest_v1/media/math/render/svg/4433f0f10a02b0242e08dce6a9914a5b986c3eae)
![{\ frac {d [C]} {dt}} = k_ {2} [B]](https://wikimedia.org/api/rest_v1/media/math/render/svg/fe4910c6054aef88e763916ed2fd70380eb01eb3)
![{\ frac {d [B]} {dt}} = 0](https://wikimedia.org/api/rest_v1/media/math/render/svg/22444fba87ae3f74b81c508b8f9d525489e5393a)
![{\ frac {d [B]} {dt}} = 0 = k_ {1} [A] -k_ {2} [B] \ Rightarrow \; [B] = {\ frac {k_ {1}} {k_ {2}}} [A]](https://wikimedia.org/api/rest_v1/media/math/render/svg/31059cc8f70a8eb11364cb0ee00ea13adceb1551)
![{\ frac {d [C]} {dt}} = k_ {1} [A]](https://wikimedia.org/api/rest_v1/media/math/render/svg/1e40aa9c96552b49d5cd3a59dbd3fa05a99e0d05)
![[C] = [A] _ {0} \ left (1-e ^ {{- k_ {1} t}} \ right)](https://wikimedia.org/api/rest_v1/media/math/render/svg/4a86aa268dc8497f8f07a9c995bdb6a13da42165)