1-chloro-1,1-difluoroethane
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
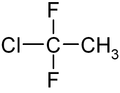
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 1-chloro-1,1-difluoroethane | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 2 H 3 ClF 2 | |||||||||||||||
| Brief description |
colorless gas with a faint odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 100.50 g mol −1 | |||||||||||||||
| Physical state |
gaseous |
|||||||||||||||
| density |
4.72 kg m −3 |
|||||||||||||||
| Melting point |
−130.8 ° C |
|||||||||||||||
| boiling point |
−9.6 ° C |
|||||||||||||||
| Vapor pressure |
0.29 M Pa (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| MAK |
|
|||||||||||||||
| Toxicological data | ||||||||||||||||
| Global warming potential |
2345 (based on 100 years) |
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
1-chloro-1,1-difluoroethane is a chemical compound that does not occur in nature , a colorless, slightly ethereal-smelling gas that is heavier than air. It belongs to the group of partially halogenated chlorofluorocarbons , damages the ozone layer and is a greenhouse gas .
Properties and toxicology
The gas is extremely flammable and forms explosive mixtures with air. It is also dangerous because it can accumulate unnoticed in enclosed spaces. In a laboratory study with rats, no toxic effects of the substance could be demonstrated; In a study with mice and rats, toxic effects only occurred at extremely high doses (inhalation of 1.7 and 2 kg within 2 and 4 hours, respectively).
Extraction
Chlorodifluoroethane industrially by reacting vinylidene chloride with hydrogen fluoride or by fluorination of 1,1,1-trichloroethane prepared.
use
Chlorodifluoroethane is used as a refrigerant and, due to this use, can be detected in small quantities in the air worldwide. It is also the starting material for the production of vinylidene fluoride and thus for the plastic polyvinylidene fluoride . In addition, chlorodifluoroethane can replace trichlorofluoromethane, which is far more damaging to the ozone layer, as a blowing agent for the production of foam .
Individual evidence
- ↑ a b c d e f g h Entry on 1-chloro-1,1-difluoroethane in the GESTIS substance database of the IFA , accessed on February 1, 2016(JavaScript required) .
- ↑ Swiss Accident Insurance Fund (Suva): Limits - Current MAK and BAT values (search for 75-68-3 or 1-chloro-1,1-difluoroethane (R 142b) ), accessed on September 30, 2019.
- ↑ a b c Toxicometric Parameters of Industrial Toxic Chemicals Under Single Exposure. Izmerov, NF, et al., Moscow, Center of International Projects, GKNT, Pg. 53, 1982 .
- ↑ G. Myhre, D. Shindell et al .: Climate Change 2013: The Physical Science Basis . Working Group I contribution to the IPCC Fifth Assessment Report. Ed .: Intergovernmental Panel on Climate Change . 2013, Chapter 8: Anthropogenic and Natural Radiative Forcing, pp. 24-39; Table 8.SM.16 ( PDF ).
- ↑ JA Seckar et al .: Toxicological evaluation of hydrochlorofluorocarbon 142b. Food Chem Toxicol. 24/3/ 1986 pp 237-40. PMID 3957176
- ↑ US Patent 5159126
- ↑ US Patent 4996378

