Chlorosulfonyl isocyanate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
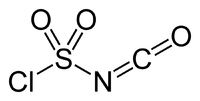
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Chlorosulfonyl isocyanate | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | ClSO 2 NCO | |||||||||||||||
| Brief description |
colorless liquid with a pungent odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 141.53 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
1.63 g cm −3 |
|||||||||||||||
| Melting point |
−44 ° C |
|||||||||||||||
| boiling point |
106 ° C |
|||||||||||||||
| Vapor pressure |
25.6 hPa (20 ° C) |
|||||||||||||||
| solubility |
|
|||||||||||||||
| Refractive index |
1.447 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Chlorosulfonyl isocyanate is a chemical compound from the isocyanate group . It was first synthesized in 1952 by Roderich Graf and published in 1956.
Extraction and presentation
Chlorosulfonyl isocyanate can be obtained by reacting cyanogen chloride with sulfur trioxide .
properties
Chlorosulfonyl isocyanate is a flammable, hardly flammable, highly volatile, colorless liquid that smokes in air and has a pungent odor, which decomposes in water and ethanol with violent to explosive reactions. It decomposes at temperatures above 300 ° C. The compound is the most reactive isocyanate known. The angle between Cl-S and N = C is 94 ° and the NCO group is not linear.
use
Chlorosulfonyl isocyanate is used to make other chemical compounds. It converts amines , alcohols and acids into N -chlorosulfonylureas, -urethanes etc.
Individual evidence
- ↑ a b c d e f g h i j Entry on chlorosulfonyl isocyanate in the GESTIS substance database of the IFA , accessed on January 10, 2017(JavaScript required) .
- ↑ a b c Entry on N-carbonylsulfamoyl chloride. In: Römpp Online . Georg Thieme Verlag, accessed on November 18, 2016.
- ↑ Data sheet Chlorosulfonyl isocyanate, Lonza quality, 99.0-100.3% (w / w) (T) from Sigma-Aldrich , accessed on November 18, 2016 ( PDF ).
- ↑ Data sheet chlorosulfonyl isocyanate (PDF) from Merck , accessed on November 18, 2016.
- ↑ Jerald K. Rasmussen, Alfred Hassner: Recent developments in the synthetic uses of chlorosulfonyl isocyanate. In: Chemical Reviews. 76, 1976, p. 389, doi : 10.1021 / cr60301a004 .
- ^ Roderich Graf: About the implementation of cyanogen chloride with sulfur trioxide. In: Chemical Reports. 89, 1956, p. 1071, doi : 10.1002 / cber.19560890437 .
- ↑ Graf, R. "chlorosulfonyl isocyanates" Organic Syntheses , Collected Volume 5, pages 226ff.
- ↑ Google Patents: Patent EP0294613A1 - Process and system for the continuous production of chlorosulfonyl isocyanate - Google Patents , accessed on November 18, 2016.
- ^ Durga Nath Dhar, Preeti Dhar: The Chemistry of Chlorosulfonyl Isocyanate . World Scientific, 2002, ISBN 978-981-238-081-4 , pp. 1 ( limited preview in Google Book search).



