Citrate synthase
| Citrate synthase | ||
|---|---|---|

|
||
| Fig. 1: Ribbon model of the monomer with bound oxaloacetate (magenta) and the acetyl-CoA analogue CMX (yellow) according to PDB 1CSI | ||
|
Existing structural data: s. Uniprot |
||
| Properties of human protein | ||
| Mass / length primary structure | 439 amino acids | |
| Secondary to quaternary structure | Homodimer | |
| Identifier | ||
| Gene name | CS | |
| External IDs | ||
| Enzyme classification | ||
| EC, category | 2.3.3.1 , transferase | |
| Response type | Transfer of an acyl group | |
| Substrate | Acetyl-CoA + H 2 O + oxaloacetate | |
| Products | Citrate + CoA | |
| Occurrence | ||
| Homology family | Citrate synthase | |
| Parent taxon | Creature | |
The citrate synthase is that enzyme (associated gene : CS ) comprising the condensation of oxaloacetate with acetyl-CoA to citrate catalyzed . This reaction is the first step in the citric acid cycle and therefore CS is indispensable in the metabolism of all aerobic organisms. In addition, the reaction and its reversal is part of the citrate-malate-pyruvate cycle to provide NADPH . The CS is located in the mitochondria , but the human CS gene is located in the cell nucleus .
Structurally, the CS consists of two large domains that consist almost entirely of α-helices (so-called all-α-protein ). The two domains are connected by an intermediate piece. Two types of CS are known. While citrate synthase is present as a dimer in eukaryotes , gram-positive bacteria and archaea (type I), the subunits in gram-negative bacteria (type II) form a hexamer (see figure below). In addition, one of the domains in type II is elongated compared with the same in type I.
The catalyzed reaction:
Oxaloacetate is reacted with acetyl-CoA to form citrate and CoA, whereby one water molecule is consumed. The reaction has a free enthalpy ΔG ° 'of −31.5 kJ / mol and is therefore rate-determining for the citric acid cycle.
CS is one of the few enzymes that is able to form CC bonds without the presence of a metal ion.
Reaction mechanism
The individual steps during catalysis that take place twice on the dimer are:
- After the binding of oxaloacetate, the angle between the two α domains increases by 18 °. This "opening" of the molecule seals and protects the oxaloacetate on the one hand, and on the other hand only allows the additional binding of CoA
- Deprotonation of the methyl group of acetyl-CoA, an enol is formed and is stabilized
- Nucleophilic addition of the enol to the carbonyl-C of the oxaloacetate, resulting in citryl-CoA
- Hydrolysis of citryl-CoA by a deprotonated water molecule to citrate and CoA-SH
- Return of the dimer to its original position
This mechanism was proposed by Remington and colleagues in 1982 based on crystallographic studies.
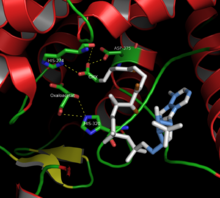
In the reaction (Fig. 2), the anion of the aspartic acid residue ASP-375 initially appears as the catalyzing base, which, together with the imidazolyl group of the histidine residue HIS-274, which has a polarizing effect on the carbonyl group of the acetyl-CoA, consists of the activated acetic acid 1 Via deprotonation, an enolate 2 to be formulated as a reactive intermediate is generated, which, analogous to an aldol addition, reacts stereospecifically with the oxaloacetate to form S- citryl-CoA 3 .
The subsequent hydrolysis of the thioester to the prochiral citrate 4 is strongly exergonic (ΔG o <−30 kJ / mol), which makes this step and thus the overall reaction catalyzed by the citrate synthase irreversible. As a product of this reaction, citrate competes with oxaloacetate for binding to the citrate synthase, so that a large concentration of citrate - despite the high exergonia of the reaction - inhibits the reaction. There is a competitive product suspension .
The catalytic center shown in Fig. 3 contains a synthetic analogue ( CMX, carboxymethyldethia-CoA ) instead of the naturally occurring acetyl-CoA , which, very similar in structure to the enol form of acetyl-CoA, replaces the citrate synthase binds, but is not suitable as a reaction partner in the sense of Claisen condensation. This acetyl-CoA analogue consequently inhibits the reaction, but makes the position of the substrates in the substrate-enzyme complex visible and thus enables a deep insight into the mechanism of this enzyme-catalyzed reaction.

The Claisen condensation mechanism for this reaction is now considered controversial (although it is still to be found in most textbooks), since the abstraction of a proton (high pKs) by an aspartate residue (low pKs) is extremely unlikely. According to more recent findings, the enzyme brings both reaction partners so close by conformational changes that the proton under consideration can neither be assigned to the methyl group nor to the aspartate residue and this promotes a transition. This is reminiscent of the mechanism of low-barrier hydrogen bonds , which can only be formed by the spatial proximity of donor and acceptor and, at −40 to −80 KJ / mol, are significantly more stable than "normal" hydrogen bonds , but this model also becomes the real one Reaction mechanism only rudimentary. It must be stated at this point that the mechanism of citrate synthase is still not fully understood, despite countless works and publications in this area.
Individual evidence
- ↑ InterPro : IPR019810 Citrate synthase active site (English)
- Jump up ↑ Voet, Donald, Judith G. Voet, and Charlotte W. Pratt. Fundamentals of Biochemistry: Life at the Molecular Level. Hoboken, NJ: Wiley, 2008.
- ^ S. Remington, G. Wiegand, R. Huber: Crystallographic refinement and atomic models of two different forms of citrate synthase at 2.7 and 1.7 A resolution. In: Journal of molecular biology . Volume 158, Number 1, June 1982, pp. 111-152, ISSN 0022-2836 . PMID 7120407 .