Creutz Taube Complex
| Structural formula | |||||||
|---|---|---|---|---|---|---|---|
|
|||||||
| General | |||||||
| Surname | Creutz Taube Complex | ||||||
| Molecular formula | C 4 H 34 N 12 Ru 2 | ||||||
| External identifiers / databases | |||||||
|
|||||||
| properties | |||||||
| Molar mass | 452.14 g mol −1 (cation) | ||||||
| safety instructions | |||||||
|
|||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||
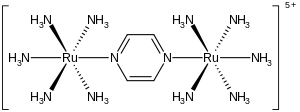
The Creutz-Taube complex is a metal complex with the formula [Ru (NH 3 ) 5 ] 2 (C 4 H 4 N 2 ) 5+ .
The cationic Creutz-Taube complex is a mixed- valent compound that has metal ions in two different oxidation states . The complex has been studied in detail to investigate the effects of electron transfer on the inner-sphere mechanism between metal centers.
The complex is named after Carol Creutz, who was the first to synthesize the complex, and her supervisor, Henry Taube , who was awarded the Nobel Prize for his work on intramolecular electron transfer.
properties
The complex consists of two ruthenium nuclei surrounded by five ammonium ligands. The nuclei are connected by the nitrogen atoms of a central, bridging pyrazine ligand. The special thing about the complex is that the ruthenium atoms have a formal charge of 2.5+. It has been shown crystallographically that the two metal centers are electronically equivalent.
A characteristic of mixed-valent complexes is strong absorption in the near infrared . The Creutz-Taube complex has an absorption maximum at 1570 nm . This absorption is described as the intermittent charge transfer band .
synthesis
The ion was first found as a hydrated salt [Ru (NH 3 ) 5 ] 2 (C 4 H 4 N 2 ) (O 3 SC 6 H 4 CH 3 ) 5 . 3H 2 O recovered.
It is produced in two steps from the pyrazine complex [Ru (NH 3 ) 5 (C 4 H 4 N 2 )] 2+ :
The Creutz-Taube complex illustrates the advantages of ruthenium complexes for the investigation of redox reactions . Ru (II) and Ru (III) ions can be converted into one another under mild conditions. The complexes with metal ions of the individual oxidation states are kinetically inert.
Web links
- Taube's Nobel Prize Lecture (PDF; 811 kB)
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ^ A b C. Creutz CH Taube: Direct Approach to Measuring the Franck-Condon Barrier to Electron Transfer Between Metal Ions. In: J. Am. Chem. Soc. 91, 1969, pp. 3988-3989; doi: 10.1021 / ja01042a072 .
- ^ H. Taube, Electron Transfer between Metal Complexes. Nobel Prize Reading, December 8, 1983, ( nobelprize.org PDF).
- ↑ U. Fürholz, S. Joss, HB Bürgi, A. Ludi: The Creutz-Taube Complex Revisited: Crystallographic Study of the Electron-Transfer Series (μ-pyrazine) decaamminediruthenium ([(NH 3 ) 5 Ru (Pyz) Ru ( NH 3 ) 5 ] n + (n = 4-6)) , in: Inorg. Chem. 24, 1985, pp. 943-948, doi: 10.1021 / ic00200a028 .
- ↑ D. Yokogawa, H. Sato, Y. Nakao, S. Sakaki: Localization or Delocalization in the Electronic Structure of Creutz-Taube-Type Complexes in Aqueous Solution , in: Inorg. Chem. 47, 2007, pp. 1966-1974, doi: 10.1021 / ic060173a .
- ^ JR Gispert: Coordination Chemistry , p. 424, 1st edition, Wiley-VCH, Weinheim, 2008, ISBN 3-527-31802-X .
![{\ mathrm {[{Ru (NH_ {3})} _ {{5}} (C_ {4} H_ {4} N_ {2})] ^ {{2 +}} + \ [{Ru (NH_ { 3})} _ {{5}} (H_ {2} O)] ^ {{2 +}} \ longrightarrow \ [{[{Ru (NH_ {3})} _ {{5}}]} _ { {2}} (C_ {4} H_ {4} N_ {2})] ^ {{4 +}} + \ H_ {2} O}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/52c7c1dee818e5a6cbc6888f2b1ba7fd0a460eb9)
![{\ mathrm {[{[{Ru (NH_ {3})} _ {{5}}]} _ {{2}} (C_ {2} H_ {4} N_ {2})] ^ {{4+ }} + \ Ag ^ {+} \ longrightarrow \ [{[{Ru (NH_ {3})} _ {{5}}]} _ {{2}} (C_ {4} H_ {4} N_ {2 })] ^ {{5 +}} + \ Ag}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/38eeffdf15259865f6e298372de0c9d6dbf44aaa)