Cyanoacetylene
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||
| General | ||||||||||
| Surname | Cyanoacetylene | |||||||||
| other names |
|
|||||||||
| Molecular formula | C 3 HN | |||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 51.05 g mol −1 | |||||||||
| Physical state |
liquid |
|||||||||
| density |
0.8167 g cm −3 (17 ° C) |
|||||||||
| Melting point |
5 ° C |
|||||||||
| boiling point |
44 ° C |
|||||||||
| solubility |
easily soluble in toluene |
|||||||||
| Refractive index |
1.3868 (25 ° C) |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||
Cyanoacetylene is an organic compound that can be seen as a monosubstituted ethyne or as a nitrile of acetylenic acid .
Occurrence
Cyanoacetylene was detected by spectroscopic methods in interstellar clouds, as well as in the tail of the comet Hale-Bopp and in the atmosphere of the Saturn moon Titan .
Presentation and extraction
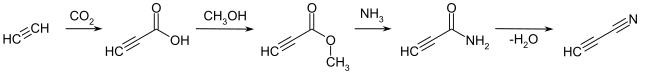
The representation of cyanoacetylene achieved by the dehydration of Acetylencarbonsäureamid in the presence of phosphorus pentoxide . The acid amide can be prepared from the acetylenecarboxylic acid via an ester stage , which is accessible by reacting ethyne with carbon dioxide .
properties
Cyanoacetylene is a low-boiling, colorless liquid. The connection solidifies below 5 ° C. Solid cyanoacetylene crystallizes in a monoclinic crystal system. The vapors are highly flammable and extremely irritating to tears. Cyanoacetylene polymerizes when exposed to UV light . No significant free radical polymerization is observed in the presence of peroxides . A 1: 1 complex is formed with ammonia via hydrogen bonds . Cyanoacetylene has a noticeable CH acidity ; H. it can be deprotonated by strong bases. The reason is the mesomerism stabilization of the anion ( −M effect of the cyano group).
use
Cyanoacrylates can be produced by ethoxycarbonylation of cyanoacetylene .
Individual evidence
- ↑ W. Dannhauser, AF Flueckinger: Dielectric Constant of Hydrogen-Bonded Liquids. I. Cyanoacetylenes. In: J. Chem. Phys. Volume 38, 1963, pp. 69-71, doi: 10.1063 / 1.1733497 .
- ↑ a b c d e f g S. Murahashi, T. Takizawa, S. Kurioka, S. Maekawa: Cyanoacetylene. Part I. The synthesis and some chemical properties. In: Nippon kagaku zassi. Volume 77, 1956, pp. 1689–1692, doi: 10.1246 / nikkashi1948.77.1689 , NASA TT F-11, 771 English translation (PDF; 359 kB).
- ↑ a b c R. J. Halter, RL Fimmen, RJ McMahon, SA Peebles, RL Kuczkowski, JF Stanton: Microwave Spectra and Molecular Structures of (Z) -Pent-2-en-4-ynenitrile and Maleonitrile. In: J. Am. Chem. Soc. Volume 123, 2001, pp. 12353-12363, doi: 10.1021 / ja011195t .
- ↑ NM Szeverenyi, RR Vold, RL Vold: Mechanisms of nuclear magnetic relaxation in cyanoacetylene. In: Chem. Phys. Volume 18, 1976, pp. 23-30, doi: 10.1016 / 0301-0104 (76) 87034-6
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-124.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ^ HB Niemann et al .: The abundances of constituents of Titan's atmosphere from the GCMS instrument on the Huygens probe. In: Nature . Volume 438, 2005, pp. 779-784, doi: 10.1038 / nature04122 .
- ↑ U. Melamed, B.-A. Feit: Reaction of acrylonitrile with benzophenone via the derived vinyl carbanion. In: J. Org. Chem. Volume 48, 1983, pp. 1928-1931, doi: 10.1021 / jo00159a037 .
- ↑ Skosarewski: In: Chem. Zentralbl. Volume 75, 1904, p. 1025.
- ^ G. Oddo: in Gazzetta Chimica Italiana. Volume 38, 1908, pp. 627-633.
- ^ FV Shallcross, GB Carpender: The crystal structure of cyanoacetylene. In: Acta Cryst. Volume 11, 1958, pp. 490-496, doi: 10.1107 / S0365110X58001365 .
- ^ DW Clarke, JP Ferris: Titan Haze: Structure and Properties of Cyanoacetylene and Cyanoacetylene – Acetylene Photopolymers. In: Icarus. Volume 127, 1997, pp. 158-172, doi: 10.1006 / icar.1996.5667 .
- ↑ N. Piétri, B. Sessouma, F. Borget, T. Chiavassa, I. Couturier-Tamburelli: Cyanoacetylene (HC3N) and ammonia (NH3) complexes: A DFT theoretical and experimental study. In: Chem. Phys. Volume 400, 2012, pp. 98-102, doi: 10.1016 / j.chemphys.2012.03.002 .
- ↑ T. Ohara, T. Sato, N. Shimizu, G. Prescher, H. Schwind, O. Weiberg, K. Marten, H. Greim: Acrylic Acid and Derivatives. In: Ullmann's Encyclopedia of Industrial Chemistry . Wiley-VCH Verlag, Weinheim 2006, doi : 10.1002 / 14356007.a01_161.pub2