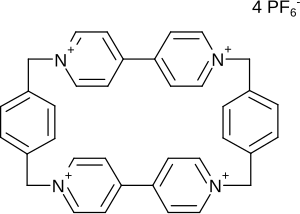
Cyclobis (paraquat-p-phenylene)
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||
| General | |||||||||||||
| Surname | Cyclobis (paraquat-p-phenylene) | ||||||||||||
| other names |
5,12,19,26-Tetraazoniaheptacyclo [24.2.2.2 2.5 .2 7.10 .2 12.15 .2 16.19 .2 21.24 ] tetraconta-1 (28), 2,4,7, 9,12,14,16,18,21,23, 26,29,31,33,35,37,39-octadecene ( IUPAC ) |
||||||||||||
| Molecular formula | C 36 H 32 N 4 | ||||||||||||
| Brief description |
white solid |
||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | 520.663 g mol −1 | ||||||||||||
| Physical state |
firmly |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Cyclobis (paraquat-p-phenylene) (formally a derivative of paraquat ) belongs to the cyclophane class , as it consists of aromatic units that are bridged by aliphatic radicals. Cyclobis (paraquat-p-phenylene), like many other cyclophanes, is able to take up a smaller molecule (a “guest”). It plays an important role in host-guest chemistry and is perhaps the best-known cyclophane in supramolecular chemistry .
It is also called " Stoddart's blue box " because its inventor, J. Fraser Stoddart , drew electron-deficient areas in molecules blue.
synthesis
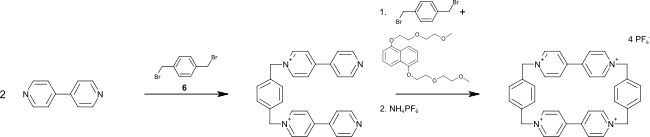
To prepare cyclobis (paraquat-p-phenylene), 4,4'-bipyridine is first mixed with 1,4-bis (bromomethyl) benzene to give 1,1 '- [1,4-phenylenebis (methylene)] bis (4,4 ′ -Bipyridine) and this reacted again in a template synthesis with 4,4′-bipyridine to give the end product. 1,5-bis (2- (2-methoxyethoxy) ethoxy) naphthalene is used as the template .
Host-guest chemistry of the CBPQT 4+
As mentioned above, CBPQT 4+ is able to accommodate a guest; this creates a host-guest complex . The interactions required for this are donor-acceptor interactions ; their strength is strongly dependent on the ability of the donor to provide π electron density. The strength of the complexation is that the stronger the π-electron donor, the stronger it is. An increase in the π system also strengthens the bond. In contrast, the kinetics of complex formation and dissociation depend on the bulkiness of the guest.
One molecule that is able to form stable complexes with CBPQT 4+ is tetrathiafulvalene (TTF). Numerous derivatives are based on this ability to complex the TTF. Variations include mechanically entrapped compounds such as catenanes and rotaxanes , molecular switches, and larger, supramolecular structures.
The charge-transfer interactions present in cyclobis (paraquat-p-phenylene) can be compared as a structural motif with the generally more frequently used hydrogen bonds , especially with regard to their directionality and their complementarity ( key-lock principle ). Charge-transfer complexes differ, however, in terms of their ease of spectroscopy and their greater tolerance to various solvents , as well as their generally lower association constant . Due to the lower association constant, significantly fewer charge transfer complexes than hydrogen bridge-based complexes are known. Other, non-covalent bonds (e.g. solvophobic forces , metal-ligand interaction ) can be used to increase the association constant; numerous structures based on this strategy are known in the literature.
As has been shown, the choice of the counterion of CBPQT 4+ has a major influence on the binding constant of the corresponding host – guest complex. CBPQT 4+ is mostly used as a hexafluorophosphate salt.
use
In order to generate catenanes, the CBPQT 4+ can serve as a template for “threading” a crown ether with a π-donor component. Then its still open ends are linked and you get two closed rings. A bistable catenane (a ring with two π donor components) is already a simple example of a molecular switch. In the present example, a cyclic ether with a TTF and a DNP unit was chosen. While the CBPQT 4+ surrounds the TTF unit in its resting position, the DNP unit is more stable as soon as the TTF is (reversibly) oxidized . In this case, the ring rotates around itself due to the Coulomb repulsion until the CBPQT 4+ can enclose the DNP unit. A reverse movement takes place as soon as the TTF unit is reduced again. This first example, which has proven its general feasibility, has been followed by numerous others.
Modifications
Numerous derivatives of the CBPQT 4+ have been developed. In this way, an enlarged version of the molecule is also possible; in the literature this is referred to as Ex n Box 4+ , where n is the number of the p - phenylene rings (n = 0-3). These variants with a larger opening are able to accommodate molecules of different sizes. Based on the charge transfer complexation of the CBPQT 4+ , numerous supramolecular structures have been created, including fibril gels , micelles , vesicles , nanotubes , foldamers and liquid crystalline phases. In analogy to biological systems that are assembled into supramolecular structures through hydrogen bonds, charge-transfer complexation is an alternative here.
Individual evidence
- ↑ a b Masumi Asakawa, Wim Dehaen, Gerrit L'abbé, Stephan Menzer, Jan Nouwen, Françisco M. Raymo, J. Fraser Stoddart, David J. Williams: Improved Template-Directed Synthesis of Cyclobis (paraquat-phenylene) . In: The Journal of Organic Chemistry . tape 61 , no. January 26 , 1996, ISSN 0022-3263 , pp. 9591-9595 , doi : 10.1021 / jo961488i .
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ a b Jonathan C. Barnes, Michal Juríček, Nicolaas A. Vermeulen, Edward J. Dale, J. Fraser Stoddart: Synthesis of ExnBox Cyclophanes . In: The Journal of Organic Chemistry . tape 78 , no. 23 , December 6, 2013, p. 11962-11969 , doi : 10.1021 / jo401993n .
- ↑ Jerry L. Atwood, Jonathan W. Steed: Supramolecular chemistry . Wiley, Hoboken, NJ 2013, ISBN 978-1-118-68150-3 .
- ↑ a b Mogens Brøndsted Nielsen, Jan Oskar Jeppesen, Jesper Lau, Christian Lomholt, Dorthe Damgaard, Jens Peter Jacobsen, Jan Becher, J. Fraser Stoddart: Binding Studies between Tetrathiafulvalene Derivatives and cyclobis (paraquat-phenylene) . In: The Journal of Organic Chemistry . Vol. 66, No. 10 , p. 3559-3563 , doi : 10.1021 / jo010173m .
- ↑ a b Anindita Das, Suhrit Ghosh: Supramolecular Assemblies by Charge-Transfer Interactions between Donor and Acceptor Chromophores . In: Angewandte Chemie International Edition . Vol. 53, No. 8 , February 17, 2014, p. 2038–2054 , doi : 10.1002 / anie.201307756 .
- ↑ Sissel S. Andersen, Morten Jensen, Anne Sørensen, Eigo Miyazaki, Kazuo Takimiya, Bo W. Laursen, Amar H. Flood, Jan O. Jeppesen: Anion effects on the cyclobis (paraquat-p-phenylene) host . In: Chemical Communications . Vol. 48, No. 42 , p. 5157 , doi : 10.1039 / c2cc31225e .
- ↑ Ognjen Š. Miljanić, William R. Dichtel, Shahab Mortezaei, J. Fraser Stoddart: Cyclobis (paraquat-phenylene) -Based [2] Catenanes Prepared by Kinetically Controlled Reactions Involving Alkynes . In: Organic Letters . Vol. 8, No. 21 , p. 4835-4838 , doi : 10.1021 / ol061864d .
- ^ Albert C. Fahrenbach, Scott C. Warren, Jared T. Incorvati, Alyssa-Jennifer Avestro, Jonathan C. Barnes, J. Fraser Stoddart, Bartosz A. Grzybowski: Organic Switches for Surfaces and Devices . In: Advanced Materials . Vol. 25, No. 3 , January 18, 2013, p. 331–348 , doi : 10.1002 / adma.201201912 .