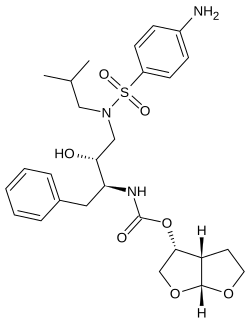
Darunavir
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Darunavir | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 27 H 37 N 3 O 7 S | |||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 547.66 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Darunavir is an antiviral agent from the group of HIV protease inhibitors , for the treatment of HIV is used.
properties
As an HIV protease inhibitor, darunavir prevents the cleavage of the viral polyprotein Gag-Pol and thus the maturation of HIV, thereby interrupting the replication cycle . Darunavir binds the viral protease of HIV with a dissociation constant of 4.5 · 10 −12 M. It binds to aspartic acids at positions 25 and 25 ', 29, 30, 30' and glycine at position 27. The dissociation constant is around two up to three powers of ten smaller than with other HIV protease inhibitors, which makes it difficult to develop resistance to darunavir through escape mutations . Darunavir is used in combination with ritonavir or cobicistat . Therapy with darunavir can avoid the toxicity of nucleoside analogues , provided that there is no resistance to HIV protease inhibitors.
Darunavir is on the World Health Organization's list of Essential Medicines . It is used to treat people infected with HIV from the age of three.
Darunavir is broken down by cytochrome P450 3A, therefore the breakdown is slowed down by inhibiting cytochrome P450 3A, e.g. B. by adding cobicistat.
Side effects
Adverse drug reactions to darunavir include disorders of the digestive tract and lipid metabolism ( cholesterol and triglycerides ). The most common side effect is skin rash (7% of patients). There are also diarrhea (2.3%), headaches (3.8%), abdominal pain (2.3%), constipation (2.3%) and vomiting (1.5%). Patients allergic to ritonavir may also have reactions to darunavir. In some patients there is a redistribution of body fat towards visceral adipose tissue .
Trade names
Trade names for darunavir are e.g. B. Prezista , Rezolsta (combination preparation with cobicistat).
literature
- JC Corrêa, DM D'Arcy, CH Serra, HR Salgado: A critical review of properties of darunavir and analytical methods for its determination. In: Critical Reviews in Analytical Chemistry. Volume 44, number 1, 2014, pp. 16-22, doi : 10.1080 / 10408347.2013.826573 , PMID 25391211 .
Individual evidence
- ↑ a b Datasheet Darunavir, ≥98% (HPLC) from Sigma-Aldrich , accessed on December 2, 2017 ( PDF ).
- ↑ a b N. M. King, M. Prabu-Jeyabalan, EA Nalivaika, P. Wigerinck, MP de Béthune, CA Schiffer: Structural and thermodynamic basis for the binding of TMC114, a next-generation human immunodeficiency virus type 1 protease inhibitor. In: Journal of Virology. Volume 78, number 21, November 2004, pp. 12012-12021, doi : 10.1128 / JVI.78.21.12012-12021.2004 , PMID 15479840 , PMC 523255 (free full text).
- ↑ G. Leonis, Czyżnikowska, G. Megariotis, H. Reis, MG Papadopo: Computational studies of darunavir into HIV-1 protease and DMPC bilayer: necessary conditions for effective binding and the role of the flaps. In: Journal of Chemical Information and Modeling. Volume 52, Number 6, June 2012, pp. 1542-1558, doi : 10.1021 / ci300014z , PMID 22587384 .
- ↑ D. Li, Y. Zhang, RN Zhao, S. Fan, JG Han: Investigation on the mechanism for the binding and drug resistance of wild type and mutations of G86 residue in HIV-1 protease complexed with Darunavir by molecular dynamic simulation and free energy calculation. In: Journal of Molecular Modeling. Volume 20, number 2, February 2014, p. 2122, doi : 10.1007 / s00894-014-2122-y , PMID 24526384 .
- ↑ JM Llibre, A. Imaz, B. Clotet: From TMC114 to darunavir: five years of data on efficacy. In: AIDS Reviews. Volume 15, Number 2, 2013 Apr-Jun, pp. 112-121, PMID 23708741 .
- ^ A b I. Pérez Valero, A. González-Baeza, ML Montes Ramírez: Central nervous system penetration and effectiveness of darunavir / ritonavir monotherapy. In: AIDS Reviews. Volume 16, Number 2, 2014 Apr-Jun, pp. 101-108, PMID 24937204 .
- ↑ A. Capetti, MV Cossu, G. Rizzardini: Darunavir / cobicistat for the treatment of HIV-1: a new era for compact drugs with high genetic barrier to resistance. In: Expert Opinion on Pharmacotherapy. Volume 16, number 17, 2015, pp. 2689-2702, doi : 10.1517 / 14656566.2015.1109632 , PMID 26612518 .
- ^ WHO Model List of Essential Medicines (19th List) . In: World Health Organization . April 2015. Archived from the original on December 13, 2016. Retrieved on December 8, 2016.
- ↑ GM Keating: Darunavir: A Review in Pediatric HIV-1 Infection. In: Pediatric Drugs. Volume 17, number 5, October 2015, pp. 411-421, doi : 10.1007 / s40272-015-0146-0 , PMID 26323490 .
- ↑ O. Putcharoen, T. Do, A. Avihingsanon, K. Ruxrungtham: Rationale and clinical utility of the darunavir-cobicistat combination in the treatment of HIV / AIDS. In: Drug Design, Development and Therapy. Volume 9, 2015, pp. 5763-5769, doi : 10.2147 / DDDT.S63989 , PMID 26566368 , PMC 4627402 (free full text).
- ↑ ED Deeks: Darunavir: a review of its use in the management of HIV-1 infection. In: Drugs. Volume 74, Number 1, January 2014, pp. 99-125, doi : 10.1007 / s40265-013-0159-3 , PMID 24338166 .