Decanenitrile
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
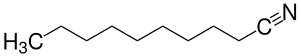
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Decanenitrile | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 10 H 19 N | ||||||||||||||||||
| Brief description |
white to yellowish liquid with an ammonia-like odor |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 153.27 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
0.82 g cm −3 |
||||||||||||||||||
| Melting point |
−15 ° C |
||||||||||||||||||
| boiling point |
243 ° C |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| Refractive index |
1.4300 (20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
Decanenitrile is a chemical compound from the group of nitriles .
Extraction and presentation
Decanenitrile can be obtained by reacting 1-bromononane with sodium cyanide in dimethyl sulfoxide (DMSO) as a solvent as part of a nucleophilic substitution .
A second-order nucleophilic substitution takes place and, when polar aprotic solvents (e.g. DMSO) are used, yields of decanenitrile of about 93%.
properties
Decanenitrile is a flammable, hardly inflammable, light-sensitive, white to yellowish liquid with an ammonia- like odor, which is practically insoluble in water.
Web links
Commons : Decannitril - Collection of images, videos and audio files
Individual evidence
- ↑ a b c d e f g h i Entry on decanenitrile in the GESTIS substance database of the IFA , accessed on January 2, 2019(JavaScript required) .
- ^ William M. Haynes: CRC Handbook of Chemistry and Physics . CRC Press, 2016, ISBN 978-1-4987-5429-3 , pp. 12 ( limited preview in Google Book search).
- ↑ Data sheet Decanonitrile, 98% from AlfaAesar, accessed on January 2, 2019 ( PDF )(JavaScript required) .
- ↑ a b K. Peter C. Vollhardt, Neil E. Schore, Katrin-M. Roy: Organic chemistry . Ed .: Holger Butenschön. 5th edition. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim 2011, ISBN 978-3-527-32754-6 , p. 1058 ( limited preview in Google Book search).


