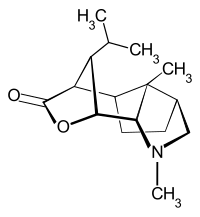
Dendrobin
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||
| General | |||||||||||||
| Surname | Dendrobin | ||||||||||||
| other names |
|
||||||||||||
| Molecular formula | C 16 H 25 NO 2 | ||||||||||||
| Brief description |
colorless needles or prisms |
||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | 263.34 g mol −1 | ||||||||||||
| Physical state |
firmly |
||||||||||||
| Melting point |
135 ° C |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Dendrobin is one of the dendrobium alkaloids found in orchids of the genus Dendrobium . The dried stems of Dendrobium nobile are used as "Chin-Shih-Hu" in traditional Chinese medicine as a fever and tonic.
The compound was first isolated in 1932 by Hideki Suzuki by extraction and crystallization . The structure was elucidated independently in 1964 by three research groups. In 1972, the first total synthesis leading to a racemic mixture of dendrobin was described and in 2012 an asymmetric total synthesis was published.
properties
Dendrobin crystallizes from diethyl ether in the form of colorless needles or prisms, sometimes also as board-like crystals. The compound can be sublimated , has a bitter taste and is soluble in organic solvents . The hydrochloride of the compound can be separated out by passing hydrogen chloride into the ethereal solution and is easily soluble in water.
structure
The compound has a tetracyclic ring system with a total of seven stereocenters . In addition to the central cyclohexane ring, the molecule contains a cyclopentane ring, a pyrrolidine unit and a butyrolactone structural element.
synthesis
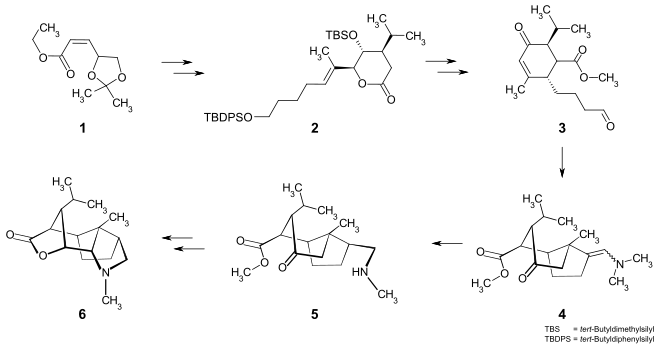
The enantioselective total synthesis of (-) - dendrobin can be achieved in 18 steps. Starting from the α, β-unsaturated ester 1 , the cyclohexane ring is first built up via the lactone 2 with an Ireland-Claisen rearrangement . The aldehyde group of the cyclohexenone derivative 3 is converted into an enamine , which reacts in the sense of an intramolecular Michael addition to form the bicyclic compound 4 . After hydrogenation to the amino compound 5 , the pyrrolidine ring is built up via regioselective bromination and subsequent nucleophilic substitution . The total synthesis to (-) - dendrobin 6 is completed by a reductive lactonization.
Individual evidence
- ↑ a b c Hideki Suzuki, Ichiro Keimatsu: About the alkaloids of the Chinese drug "Chin-Shih-Hu". (II. Communication). About dendrobin. (I) . In: Yakugaku Zasshi . tape 52 , no. 12 , April 2, 2012, p. 1049-1060 , doi : 10.1248 / yakushi1881.52.12_1049 .
- ↑ a b c d Safety data sheet Dendrobine. Santa Cruz Biotechnology, Inc., May 2, 2015, accessed June 23, 2020 .
- ^ W. Steglich, B. Fugmann, S. Lang-Fugmann: RÖMPP Lexikon Naturstoffe , 1st edition, Thieme-Verlag, 1997, p. 173 restricted preview in the Google book search
- ↑ Tadamasa Onaka, Susumu Kamata, Takashi Maeda, Yutaka Kawazoe, Mitsutaka Natsume, Toshihiko Okamoto, Fumihiko Uchimaru, Masao Shimizu: The Structure of Dendrobine . In: CHEMICAL & PHARMACEUTICAL BULLETIN . tape 12 , no. 4 , 1964, pp. 506 , doi : 10.1248 / cpb.12.506 .
- ↑ Y. Inubushi, J. Nakano: Structure of dendrine . In: Tetrahedron Letters . tape 6 , no. 31 , 1965, p. 2723 , doi : 10.1016 / S0040-4039 (01) 99532-2 .
- ↑ Shosuke Yamamura, Yoshimasa Hirata: Structures of nobiline and dendrobine . In: Tetrahedron Letters . tape 5 , no. January 2 , 1964, p. 79 , doi : 10.1016 / S0040-4039 (00) 90333-2 .
- ↑ Kiyoyuki Yamada, Masaaki Suzuki, Yoshihiro Hayakawa, Kouzou Aoki, Hitoshi Nakamura, Hiroshi Nagase, Yoshimasa Hirata: Total synthesis of (+ -) - dendrobine . In: Journal of the American Chemical Society . tape 94 , no. 23 November 1972, p. 8278 , doi : 10.1021 / ja00778a083 .
- ↑ Lukas M. Kreis, Erick M. Carreira: Total Synthesis of ( -) - Dendrobine . In: Angewandte Chemie . tape 124 , no. 14 , April 2, 2012, p. 3492 , doi : 10.1002 / anie.201108564 .
- ↑ Lukas Kreis: Total synthesis of (-) - Dendrobine and Cobalt catalyzed Alkyl Heck reactions . Dissertation ETH No. 20449. ETH, Zurich 2012, doi : 10.3929 / ethz-a-7335119 .