Deserpidine
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
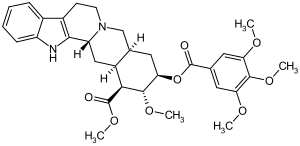
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Deserpidine | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 32 H 38 N 2 O 8 | |||||||||||||||||||||
| Brief description |
colorless crystals |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 578.65 g mol −1 | |||||||||||||||||||||
| Melting point | ||||||||||||||||||||||
| pK s value |
6.68 |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Deserpidin is a natural product from the group of alkaloids , more precisely the Rauwolfia alkaloids . It is structurally very similar to reserpine ; it differs only in the absence of a –OCH 3 group on the indole partial structure.
Occurrence and extraction
Deserpidin is found in plants of the genus Rauvolfia and was first extracted from the Indian snake root in the early 20th century .
Deserpidine is synthetically accessible from reserpine.
Properties and use
The antihypertensive properties of deserpidine have been proven in numerous studies. A neuroleptic effect could also be demonstrated.
Individual evidence
- ↑ a b c d Entry on deserpidine. In: Römpp Online . Georg Thieme Verlag, accessed on June 6, 2017.
- ↑ a b c d S. Gangolli: The Dictionary of Substances and Their Effects: EJ. Royal Society of Chemistry, 1999, ISBN 978-0-854-04823-6 , p. 89 ( limited preview in Google book search).
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ SC Fulton, MD Healy: Comparison of the effectiveness of deserpidine, reserpine, and alpha-methyltyrosine on brain biogenic amines. In: Fed Proc. (1976), Volume 35, Issue 14, pp. 2558-2562. PMID 11134 .
- ↑ G. Varchi, A. Battaglia, C. Samori, E. Baldelli, B. Danieli, G. Fontana, A. Guerrini, E. Bombardelli: Synthesis of deserpidine from reserpine. In: Journal of natural products. Volume 68, Number 11, November 2005, pp. 1629-1631, doi : 10.1021 / np050179x , PMID 16309312 .
- ↑ PY Hatt: [Clinical trials of an antihypertensive drug, a combination of methylclothiazide and deserpidine]. In: Clinique. Volume 60, Number 613, November 1965, pp. 749-754, PMID 5859341 .
- ↑ HW Kimmerling: Methyclothiazide and deserpidine in the treatment of the elderly hypertensive. In: Current therapeutic research, clinical and experimental. Volume 9, Number 2, February 1967, pp. 75-78, PMID 4962534 .
