Deoxypipradrol
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
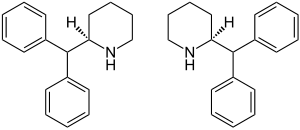
|
||||||||||||||||
| ( R ) -form (left) and ( S ) -form (right) 1: 1 mixture ( racemate ) |
||||||||||||||||
| General | ||||||||||||||||
| Non-proprietary name | Deoxypipradrol | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 18 H 21 N | |||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| Drug information | ||||||||||||||||
| Drug class | ||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 251.37 g · mol -1 | |||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Desoxypipradrol ( 2-diphenylmethylpiperidin , 2-DPMP ) is a drug selected from the group of piperidine - derivatives , of the stimulants belongs.
History and use
Deoxypipradrol was developed and researched by the pharmaceutical company Ciba-Geigy (now Novartis ) in the 1950s. It should be used in the treatment of narcolepsy and attention deficit / hyperactivity disorder . Despite the high degree of effectiveness, the development was not pursued any further because an alternative was developed by the same company: methylphenidate . Methylphenidate was believed to be the superior agent for treating ADHD because it has a shorter duration of action and its pharmacokinetic properties are more predictable than those of deoxypipradrol. As a result, other areas of use for deoxypipradrol have been sought, for example researching a faster recovery of sensation after anesthesia .
It is believed that deoxypipradrol is contained in various over-the-counter drug mixtures - known as bath salts - as an active ingredient and as a substitute for illegal amphetamines .
Manufacturing
Deoxypipradrol is a derivative of pipradrol , from which it can be obtained by reduction. Another manufacturing process is based on the condensation of 2-bromopyridine with diphenylacetonitrile in the presence of sodium amide and subsequent catalytic hydrogenation of the pyridine ring .
Side effects
The side effects of deoxypipradrol are comparable to those of pipradrol or other stimulants. When given for therapeutic purposes, sleep disorders , tachycardia , anorexia , dry mouth , tremor , arterial hypertension , euphoria , depression and psychoses can occur. Another side effect found in rats given 130 and 260 µmol / kg over a period of 14 days is hyperglycaemia . The side effect is more pronounced in young rats than in older rats.
Legal position
- Germany
In Germany, deoxypipradrol is subject to BtMG Annex II .
- United Kingdom
In the United Kingdom , following several cases of poisoning, the import of deoxypipradrol was banned under the Open General Import License effective November 4, 2011 . The Advisory Council on the Misuse of Drugs (ACMD) recommended monitoring at the same time as inclusion in the Misuse of Drugs Act ( Annex B / class B ). 2-DPMP has been classified as a class B drug since March 28, 2012 and is therefore prohibited.
literature
- G. Bellucci: (2-Diphenylmethyl-piperidine hydrochloride and the methyl ester of 2-chloro-2-phenyl-2- (2-piperidyl) -acetic acid), drugs with waking effect in anesthesia. In: Minerva Anestesiologica , 1955 Jun, 21 (6), pp. 125-128 (Italian).
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ a b c d Les Iversen: Desoxypipradrol (2-DPMP) advice. Advisory Council on the Misuse of Drugs, September 13, 2011, p. 6.
- ↑ Entry on Desoxypipradrol. In: Römpp Online . Georg Thieme Verlag, accessed on April 8, 2016.
- ^ KL Hintze, HY Aboul-Enein, LJ Fischer: Isomeric specificity of diphenylmethylpiperidine in the production of rat pancreatic islet cell toxicity . In: Toxicology , 1977 Apr, 7 (2), pp. 133-140, PMID 324024 .
- ^ AK Chatterjee, LJ Fischer: Age-related susceptibility to the insulin-depleting action of 4-diphenylmethylpiperidine in young rats . In: Life Sci. , 1988, 43 (2), pp. 151-159, PMID 3292868 .
- ↑ Lord Henley: Government accepts ACMD's advice to schedule D2PM, 2-DPMP and phenzepam . (PDF) UK Home Office , January 26, 2012.