Diisopropylnaphthalenes
Diisopropylnaphthalenes (DIPN) are chemical compounds from the group of naphthalenes . They include the isomers 1,2-, 1,3-, 1,4-, 1,5-, 1,6-, 1,7-, 1,8-, 2,3-, 2,6- and 2,7-diisopropylnaphthalene. The technical product, which is a mixture of isomers , mainly contains 1,3-, 1,4-, 1,5-, 1,6-, 1,7-, 2,6- and 2,7-diisopropylnaphthalene, with 2 , 6- and 2,7-diisopropylnaphthalene as preferred isomers make up the major part with about 40% each.
Extraction and presentation
Diisopropylnaphthalene mixtures are mainly produced from naphthalene or monoisopropylnaphthalene by alkylation or transalkylation reactions. In one production variant, naphthalene is reacted with propene in a fixed bed reactor over an aluminosilicate catalyst at temperatures between 160 ° C and 250 ° C. The composition of the resulting isomer mixtures can be influenced by the temperature. The isomers can be separated by a combination of distillation and crystallization steps.
properties
Diisopropylnaphthalene as a technical product is a colorless and odorless liquid. 1,3- and 2,6-diisopropylnaphthalene are colorless solids.
| Diisopropylnaphthalenes | ||||||||||||
| Surname | 1.2- | 1.3- | 1.4- | 1.5- | 1.6- | 1.7- | 1.8- | 2.3- | 2.6- | 2.7- | ||
| other names |
|
|||||||||||
| Structural formula |
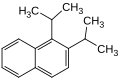
|
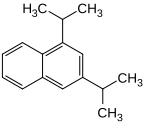
|

|

|

|

|
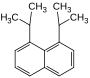
|

|

|

|
||
| CAS number | 94133-79-6 | 57122-16-4 | 24157-79-7 | 27351-96-8 | 51113-41-8 | 94133-80-9 | 24192-58-3 | 94133-81-0 | 24157-81-1 | 40458-98-8 | ||
| 38640-62-9 (mixture of isomers) | ||||||||||||
| PubChem | 92672 | 117971 | 3023652 | 90390 | 32241 | 94505 | ||||||
| Molecular formula | C 16 H 20 | |||||||||||
| Molar mass | 212.33 g mol −1 | |||||||||||
| Physical state | liquid | firmly | liquid | firmly | liquid | |||||||
| Brief description | colorless and odorless liquid (mixture of isomers) | |||||||||||
| Melting point | 46.2 ° C | 68.5 ° C; 70 ° C | ||||||||||
| <−40 ° C (mixture of isomers) | ||||||||||||
| boiling point | 90 ° C (0.8 Torr) | 309 ° C | 315 ° C 293 ° C | 311 ° C | 318 ° C | 309 ° C | 318 ° C 290-300 ° C | 319 ° C 279.3 ° C | 317 ° C 280 ° C | |||
| 290–299 ° C (mixture of isomers) | ||||||||||||
| density | 1.0073 | 0.965 | ||||||||||
| 0.96 g cm −3 (mixture of isomers) | ||||||||||||
| solubility | practically insoluble in water (0.11 mg l −1 at 25 ° C) | |||||||||||
| Refractive index | 1.564 | 1.565 (25 ° C) | 1.565 (25 ° C) | 1.5701 | ||||||||
| 1.565 (mixture of isomers) | ||||||||||||
| Heat capacity | 1.71 kJ kg −1 K −1 (mixture of isomers at 20 ° C) | |||||||||||
| Thermal conductivity | 0.12 W m −1 K −1 (mixture of isomers at 20 ° C) | |||||||||||
| Flash point | > 130 ° C (mixture of isomers) | |||||||||||
|
GHS labeling |
|
|||||||||||
| H and P phrases | no H-phrases | |||||||||||
| no EUH phrases | ||||||||||||
| no P-phrases | ||||||||||||
| Toxicological data | > 3900 mg kg −1 ( LD 50 , rat , oral ) | |||||||||||
use
Diisopropylnaphthalene is used as a substitute for polychlorinated biphenyls and as a solvent for dyes in carbonless papers. In Japan and Germany alone, 10,000 tons of the DIPN isomer mixture are produced each year. The DIPN creates problems when recycling waste paper if it is then used as packaging material in the food sector, as it is not removed during the recycling process and can be transferred to the food in the absence of additional outer packaging. At the moment (as of 2001) there are no specific health concerns about DIPN, but according to the Federal Institute for Consumer Health Protection and Veterinary Medicine, the content of DIPN should be kept as low as technically possible in the sense of the general minimization requirement. According to a study by the Federal Institute for Risk Assessment (as of 2011) , the DIPN are toxicologically harmless, as the alkyl substitution of the aromatic ring prevents ring oxidation and thus the conversion to toxic reaction products. 2,6-DIPN is functionally and structurally identical to naturally occurring plant growth regulators in potatoes and is therefore also used as a germ inhibitor for potatoes.
literature
- Gabriele Haack: Investigations into the interactions between carton-based food packaging and its filling goods: Determination and estimation of distribution coefficients between cartons and food (simulants) . Munich 2006, DNB 985511753 , urn : nbn: de: bvb: 91-diss20070104-2128347919 (dissertation, TU Munich ).
Individual evidence
- ↑ a b Robert Brzozowski, Jan Cz. Dobrowolski, Michał H. Jamróz, Wincenty Skupinski: Studies on diisopropylnaphthalene substitutional isomerism . In: Journal of Molecular Catalysis A: Chemical . tape 170 , no. 1 , May 2001, p. 95-99 , doi : 10.1016 / S1381-1169 (00) 00427-1 .
- ↑ a b c d e Brzozowski, R .; Skupiński, W .; Jamróz, WH; Skarżyński, M .; Otwinowska, H .: Isolation and identification of diisopropylnaphthalene isomers in the alkylation products of naphthalene in J. Chromatogr. A 946 (2002) 221-227, doi: 10.1016 / S0021-9673 (01) 01571-0 .
- ↑ a b c d e IUCLID Datasheet: bis (isopropyl) naphthalene ( Memento of January 2, 2014 in the Internet Archive ) of February 18, 2000.
- ↑ a b Entry on 2,6-diisopropylnaphthalenes at TCI Europe, accessed on January 10, 2012.
- ↑ a b Entry on 2,6-diisopropylnaphthalene at ChemicalBook , accessed January 9, 2012.
- ↑ a b c d e f g h Collin, G .; Höke, H .; Greim, H .: Naphthalene and Hydronaphthalenes in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim 2012, doi : 10.1002 / 14356007.a17_001.pub2 .
- ↑ a b Braude, EA; Jackman, LM; Linstead, RP; Lowe, G .: Hydrogen transfer. Part XII. Dehydrogenation of “blocked” hydroaromatic compounds by quinones in J. Chem. Soc. , 1960 , pp. 3123-3132, doi: 10.1039 / JR9600003123 .
- ↑ a b c d e f g h Bouviera, C .; Reumkens, N .; Buijs, W .: Separation of diisopropylnaphthalene isomers in J. Chromatogr. A 1216 (2009) 6410-6416, doi: 10.1016 / j.chroma.2009.07.006 .
- ↑ a b c d e f g h Patent US4950824 : Reacting monoisopropylnaphthalene with propylene and transalkylation of mixture formed. Filed on Aug. 2, 1989 , published on August 21, 1990 , Applicant: Chiyoda Corporation , NKK Corporation, inventor Yoshimi Shiroto et al ..
- ↑ Egorow et al .: Zhurnal Fizicheskoi Khimii 34 (1960) 888,889, (English edition) p. 422.
- ↑ Entry on 2,7-diisopropylnaphthalene at ChemicalBook , accessed January 9, 2012.
- ↑ Hueckel, W .; Cramer, R .; Läufer, S .: Reductions in liquid ammonia, XIII Reduction and reducing methylation of 2-methyl- and 1,2.3-trimethylnaphthalene; Isopropylation of naphthalene in: Liebigs Ann. Chem. , 1960 , 630 , pp. 89-104, doi: 10.1002 / jlac.19606300112 .
- ^ Carl L. Yaws: Thermophysical Properties of Chemicals and Hydrocarbons . William Andrew, 2014, ISBN 978-0-323-29060-9 , pp. 332 ( limited preview in Google Book search).
- ↑ a b c Entry on Diisopropylnaphthalene in the Hazardous Substances Data Bank , accessed July 27, 2012.
- ↑ Zukerwanik, Terentjewa; in: Chem. Zentralblatt , 1938 , 109 , p. 579.
- ↑ Page no longer available , search in web archives: Current topic: Diisopropylnaphthalene (DIPN) (PDF; 382 kB), accessed on January 5, 2012.
- ↑ BFR: Report on the 109th meeting of the commission / expert group for the health assessment of plastics and other materials within the framework of the Food and Commodities Act of the Federal Institute for Consumer Health Protection and Veterinary Medicine (plastics commission / expert group of the BgVV) on 25. / 26. April 2001 in Berlin (PDF; 84 kB)
- ↑ Mineral oil derivatives from food packaging - current status from a toxicological point of view (PDF; 241 kB)
- ↑ EPA: 2,6-Diisopropylnaphthalenes