Dimefuron
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
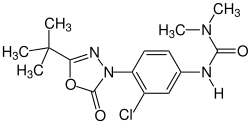
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Dimefuron | |||||||||||||||
| other names |
N '- [4- (5- tert -Butyl-2-oxo-1,3,4-oxadiazol-3 (2 H ) -yl) -3-chlorophenyl] - N , N -dimethylurea |
|||||||||||||||
| Molecular formula | C 15 H 19 ClN 4 O 3 | |||||||||||||||
| Brief description |
colorless and odorless solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 338.79 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
193 ° C |
|||||||||||||||
| solubility |
practically insoluble in water (16 mg l −1 at 20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Dimefuron is a chemical compound from the group of phenylureas that is used as a herbicide .
synthesis
Dimefuron is produced from 3-chloro-4-nitroaniline through reactions with phosgene , dimethylamine and sodium nitrite .
use
Dimefuron is mainly absorbed through the roots and is one of the selective soil herbicides. It inhibits the electron transport in photosystem II of photosynthesis, which interrupts the Hill reaction .
The selectivity of the urea derivatives is based on their distribution in the soil. They are not very soluble in water and are strongly adsorbed by the soil, which keeps them in the uppermost soil horizon. Weeds with their shallow roots that only reach into the upper soil layer absorb toxic quantities and die. The deeper-rooted crops, on the other hand, only absorb harmless quantities. However, in the event of heavy rainfall , the herbicide can still be shifted to deeper soil layers. Few plant species like cotton metabolize urea derivatives quickly and do not depend on the physical selectivity mechanism.
Dimefuron is used - often in combination with carbetamide - in cotton, peanut, rapeseed, alfalfa and wheat cultivation .
Admission
In 2002, Dimefuron was not included in the list of active ingredients for plant protection products approved in the EU . No preparation with this active ingredient is approved in Germany, Austria and Switzerland.
Individual evidence
- ↑ a b c Bahadir, Parlar, Spiteller: Springer Umweltlexikon . Springer, 2000, ISBN 3-540-23480-2 , pp. 322 ( limited preview in Google Book search).
- ↑ a b c d Entry on Dimefuron. In: Römpp Online . Georg Thieme Verlag, accessed on July 5, 2014.
- ↑ a b Datasheet Dimefuron at Sigma-Aldrich , accessed on April 30, 2017 ( PDF ).
- ↑ Thomas A. Unger: Pesticide synthesis handbook . 1996, ISBN 978-0-8155-1401-5 , pp. 479 .
- ^ JL Glasgow, JW Dicks: The basis of field tolerance of field bean and pea to dimefuron . In: Weed Research . tape 20 , no. 1 , February 1980, p. 17-23 , doi : 10.1111 / j.1365-3180.1980.tb00036.x .
- ↑ Regulation (EC) No. 2076/2002 of the Commission of November 20, 2002 (PDF) extending the deadline according to Article 8 (2) of Council Directive 91/414 / EEC and on the non-inclusion of certain active substances in Annex I of this Directive and the revocation of the approval of plant protection products with these active substances.
- ↑ General Directorate Health and Food Safety of the European Commission: Entry on Dimefuron in the EU pesticide database; Entry in the national registers of plant protection products in Switzerland , Austria and Germany ; accessed on February 22, 2016.

