Disodium phenyldibenzimidazole tetrasulfonate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
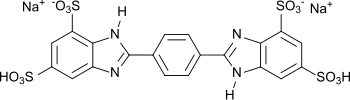
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Disodium phenyldibenzimidazole tetrasulfonate | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 20 H 12 N 4 Na 2 O 12 S 4 | |||||||||||||||
| Brief description |
fine yellow powder |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 674.57 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
> 280 ° C |
|||||||||||||||
| solubility |
soluble in water (12% at 20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Disodium phenyldibenzimidazole tetrasulfonate (DPDT) is a water-soluble broad-spectrum UV absorber with an absorption maximum λmax in the UV-A range at 335 nm.
Manufacturing
Purified 1,2-phenylenediamine is dissolved in concentrated sulfuric acid and chlorosulfonic acid is added dropwise. The mixture is heated briefly to 120 ° C. with evolution of hydrogen chloride , then cooled to 70 ° C. and terephthalic acid is added. After further heating to 180 ° C. with evolution of HCl, the mixture is cooled to 80 ° C. and poured onto ice, the tetrasulfonic acid precipitating in crystalline form.
For cleaning, the acid is dissolved in sodium hydroxide solution and boiled with activated charcoal . When sulfuric acid is added, DPDT is obtained as a symmetrical di-4,6-sulfonate in 99% purity.
Obviously, the DPDT produced according to this regulation still contains impurities that lead to discoloration in the sunscreen formulations made from it .
The reaction of o- phenylenediamine with terephthalic acid and chlorosulfonic acid at 110-120 ° C and a reaction time of 10 to 15 hours gives DPDT in 98% purity after repeated recrystallization. The disodium phenyldibenzimidazole tetrasulfonate obtained in this way contains small amounts of tetrasulfonic acid, which is asymmetrically sulfonated on one side in the 4,5-position, and trisulfonic acid, which are not problematic impurities, as by-products
properties
Disodium phenyldibenzimidazole tetrasulfonate is an odorless, yellow, finely crystalline, strongly hygroscopic solid which, as the disodium salt of the underlying tetrasulfonic acid, dissolves up to 12% in water.
When adding bases, such as. B. triethanolamine (TEA), however, the tetrasulfonate dissolves very well in water at room temperature.
Solubilities from DPDT solvent at 20 ° C Ethanol 96% mineral oil Isopropyl myristate Caprylic / capric acid triglycerides Water (as free acid) Water (as TEA salt) Water (as sodium salt) solubility in % <0.1 insoluble insoluble insoluble 1 22nd 12
As a triethanolamine salt in water, disodium phenyldibenzimidazole tetrasulfonate has UV absorption under standard conditions (1% solution, 1 cm layer thickness) E 1% / 1 cm of approx. 770 at a wavelength of approx. 335 nm in the UVA II range (320–340 nm) . The absorption spectrum of DPDT is relatively broad and extends from the shorter-wave UVB range (290–320 nm) to the UVA I range (340–400 nm)
Applications
Disodium phenyldibenzimidazole tetrasulfonate was approved as a sunscreen in Europe in 2000. The substance is on the market in sun protection preparations in concentrations of up to 10% in the European Union, Australia and New Zealand, South Africa, South Korea, China, the ASEAN and Mercosur countries; there is no approval in the USA or Japan.
In combination with oil-soluble UV B filters, such as B. Octocrilen or Enzacamen causes DPDT an increase of the sun protection factor by approx. 40%.
As a relatively large (MW> 600) and polar tetrasulfonic acid derivative, DPDT penetrates the skin only very slightly and, due to its water solubility, it can be formulated not only in O / W emulsions, but also in clear, aqueous sun protection preparations such as gels and sprays.
Trade names
Neo Heliopan AP
Individual evidence
- ↑ Entry on DISODIUM PHENYL DIBENZIMIDAZOLE TETRASULFONATE in the CosIng database of the EU Commission, accessed on December 29, 2019.
- ↑ a b c d e f Symrise, UV Protection, Neo Heliopan® AP, 106796 ( Memento of the original dated December 24, 2015 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ↑ Patent EP0669323A1 : The use of benzazoles as UV absorbers, new benzazoles and a process for their production. Registered on February 13, 1995 , published on August 30, 1995 , applicant: Haarmann & Reimer GmbH, inventors: R. Pelzer, R. Langner, H. Surburg, H. Sommer, A. Krempel, R. Hopp.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ U. Osterwalder, B. Herzog, Overview of new UV-Filters, Understanding Sunscreens , Intensive Course in Dermato-Cosmetic Sciences, Brussels, 7-11 September 2015, PDF ( Memento of the original from December 24, 2015 in the Internet Archive ) Info : The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ↑ EL Martin: o-Phenylenediamine In: Organic Syntheses . 19, 1939, p. 70, doi : 10.15227 / orgsyn.019.0070 ; Coll. Vol. 2, 1943, p. 501 ( PDF ).
- ↑ Patent EP1690855A1 : Process for the production of phenylene-bis-benzimidazole-tetrasulfonic acid disodium salt. Registered on November 21, 2001 , published on August 16, 2006 , applicant: Symrise GmbH & Co. KG, inventors: R. Bertram, S. Hillers, O. Koch, H. Erfurt, G. Reinders.
- ↑ BASF: Cross-Reference List of all UV Filters used in the BASF Sunscreen Simulator ( Memento of the original from May 29, 2015 in the Internet Archive ) Info: The archive link was automatically inserted and not yet checked. Please check the original and archive link according to the instructions and then remove this notice.
- ↑ Directive 2000/6 / EG (PDF)
- ↑ Handbook of Photomedicine, MR Hamblin, Y.-Y. Huang, edit., 13. Photoprotection, CRC Press, Boca Raton, ISBN 978-1-4398-8469-0 , p. 135.
- ↑ MS Reisch: After More Than A Decade, FDA Still Won't Allow New Sunscreens . In: Chemical & Engineering News . tape 93 , no. 20 , 2015, p. 10-15 ( online ).

