Endocrocin
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
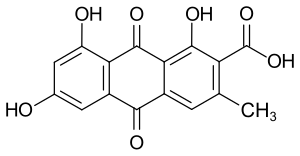
|
|||||||||||||
| General | |||||||||||||
| Surname | Endocrocin | ||||||||||||
| other names |
|
||||||||||||
| Molecular formula | C 16 H 10 O 7 | ||||||||||||
| Brief description |
orange leaflets |
||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | 314.25 g mol −1 | ||||||||||||
| Physical state |
firmly |
||||||||||||
| density |
1.715 g cm −3 |
||||||||||||
| Melting point |
340 ° C |
||||||||||||
| pK s value |
4.2 |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Endocrocin is an organic compound and belongs to the group of dyes red anthraquinone . Endocrocin belongs to the group of ergot pigments that was first elucidated, which are characterized by their increased acidity and intense color. The compound is considered a precursor to many anthraquinones .
Occurrence
Endocrocin occurs primarily in mushrooms (e.g. yellow-leaved cinnamon skin head ), cactus plants (e.g. Echinopsis cinnabarina ), molds (e.g. Aspergillus amstelodami ), lichens (e.g. the Japanese tree lichen Nephromopsis endocrocea ) and in ergot before. Due to the high content of anthraquinone dyes in ergot, it is assumed that the physiological effect of the same is significantly influenced by the dyes. Ergot contains 0.03 g · kg −1 endocrocin.
history
The research into the connection between alkaloid and dye formation, as well as the elucidation of the structures of the same, can be traced back to the work of Burchard Franck . In doing so, he discovered endocrocin as a precursor to the biosynthesis of yellow xanthone dye derivatives . Due to their very stable structure, anthraquinone dyes were accepted as end products of the metabolism of plants. Ten years after his discovery, Franck found out that they are further processed into xanthone compounds by oxidative ring opening .
See also
literature
- Klaus Roth (2010): Chemical Delicacies . 1st edition, Weinheim: Wiley-VCH, p. 92. ISBN 978-3-527-32752-2 .
Individual evidence
- ↑ a b c d e entry on endocrocin. In: Römpp Online . Georg Thieme Verlag, accessed on June 4, 2020.
- ↑ Chemsrc: 481-70-9 .
- ↑ a b c d Franck, B .: Structure and Biosynthesis of Ergot Dyes , Angewandte Chemie 1969 , 81 (8) , 269–278, doi: 10.1002 / anie.19690810802 .
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ^ A b Franck, B .: Die dyes des ergot , 8th conference of the German Society for Medicinal Plant Research October 23, 1960 , No. 4 , 420-429.
- ↑ Franck, B. & Zimmer, I .: Constitution and Synthesis of Clavorubin , Chemical Reports , 1965 , 98 (5) , 1514-1521, doi: 10.1002 / cber.19650980527.
- ^ Franck, B. & Reschke, T .: Clavoxanthin and Clavorubin, two new ergot dyes , Angewandte Chemie 1959 , 71 (12) , 407, doi: 10.1002 / anie.19590711207 .
