Falcarinol
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
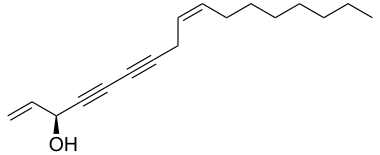
|
||||||||||
| Structural formula of (-) - falcarinol | ||||||||||
| General | ||||||||||
| Surname | Falcarinol | |||||||||
| other names |
|
|||||||||
| Molecular formula | C 17 H 24 O | |||||||||
| Brief description |
oily liquid |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 244.37 g mol −1 | |||||||||
| Physical state |
liquid |
|||||||||
| boiling point |
115 ° C (2.6 Pa ) |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Falcarinol is a naturally occurring unsaturated alcohol . It is a chiral alcohol with two carbon triple and two double bonds .
Meaning and effect
The substance that is effective against pests ( pesticide ) is contained, for example, in carrots , red ginseng ( Panax ginseng ), Saposhnikovia divaricata and common ivy and protects the roots of plants from various fungal diseases such as the Mycocentrospora leaf spots caused by Mycocentrospora acerina .
In a feeding experiment on rats, falcarinol showed a cancer-preventive effect in low doses; in addition, antibacterial , fungicidal and pain-relieving properties as well as inhibition of platelet aggregation were demonstrated. It is poisonous in larger quantities.
Falcarinol can cause allergic reactions and inflammatory skin irritation . For example, when cutting ivy , which has large amounts of natural toxins and the like. a. Contains falcarinol, light protective measures are therefore recommended.
Web links
- Birgit Buchroithner: Why do carrots work against cancer . In: Bild der Wissenschaft , February 9, 2005.
Individual evidence
- ↑ a b c d Entry on Falcarinol. In: Römpp Online . Georg Thieme Verlag, accessed on February 22, 2014.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Morten Kobæk-Larsen, Lars P. Christensen, Werner Vach, Jelmera Ritskes-Hoitinga, Kirsten Brandt: Inhibitory Effects of Feeding with Carrots or (-) - Falcarinol on Development of Azoxymethane-Induced Preneoplastic Lesions in the Rat Colon. In: Journal of Agricultural and Food Chemistry . 53, 2005, pp. 1823-1827, doi : 10.1021 / jf048519s .
- ^ S. Machado, E. Silva, A. Massa: Occupational allergic contact dermatitis from falcarinol. In: Contact Dermatitis . 47, 2002, pp. 109-125, doi : 10.1034 / j.1600-0536.2002.470210_5.x .
