Flibanserin
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
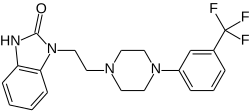
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Flibanserin | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 20 H 21 F 3 N 4 O | |||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 390,40 g · mol -1 | |||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Flibanserin is a drug that was originally developed to treat depression, but was found to be ineffective in this area, and has now been approved for the treatment of hypoactive sexual dysfunction (HSDD) in women - but its effectiveness has not been confirmed here either, which has already been the case has led to much controversy about the subject of Flibanserin (Addyi) and its alleged effect.
Pharmacological properties
Mechanism of action (pharmacodynamics)
At the molecular level, flibanserin mediates its pharmacological effects via serotonin and dopamine receptors . Flibanserin is an agonist at the serotonin receptor 5-HT 1A and an antagonist at 5-HT 2A . At the dopamine receptor D 4 , flibanserin behaves as a weak partial agonist . Through these mechanisms, flibanserin influences the release of neurotransmitters in the brain that are involved in the control of sexual functions. On the one hand it inhibits the release of the sexuality-inhibiting serotonin and on the other hand it increases the release of the sexuality-increasing neurotransmitters dopamine and norepinephrine .
Pharmacokinetics
From oral dosage forms, more than 90% of the flibanserin is absorbed into the systemic circulation in the form of the active medicinal substance or its metabolic products ( metabolites ). Its volume of distribution is about 180 liters. Flibanserin is metabolized via the cytochrome P450 enzyme system, with the isoenzyme CYP3A4 playing the most important role. A metabolism is also possible via CYP2D6 . The main products of metabolism are the pharmacologically inactive metabolites flibanserin-6-sulfate and flibanserin-6,21-disulfate. Flibanserin and its metabolites are excreted almost equally in the bile and in the urine . The terminal plasma half-life of flibanserin is about 10 hours and, including its metabolites, about 66 hours.
Analytics
The reliable qualitative and quantitative determination of flibanserin in different test materials succeeds after adequate sample preparation by coupling the HPLC with the mass spectrometry .
Clinical information
Interactions with other drugs
Since flibanserin is metabolized via the cytochrome P450 enzyme system, there is a risk of possible interaction with inhibitors and inducers of this enzyme system. For example, simultaneous intake of the CYP3A4 inhibitor ketoconazole leads to a significant increase in the level of flibanserin in the blood and impaired tolerability. An increase in flibanserin side effects was also observed with the simultaneous use of serotonin reuptake inhibitors , triptans , the birth control pill and alcohol .
Adverse effects (side effects)
In clinical studies , dizziness, tiredness, and nausea were common (> 10%). Occasionally (1 to 10%), insomnia, anxiety, dry mouth, abdominal pain, constipation, nocturnal urination, palpitation and stress were observed as side effects. The risk of syncope may be increased with flibanserin. The risk of accidents and injuries after taking flibanserin can also be increased because of its sedating side effects.
Clinical development and approval
Flibanserin was initially developed by Boehringer Ingelheim and tested in a total of seven phase III clinical studies . The two most important studies showed a statistical superiority over placebo with regard to sexual satisfaction, but no improvement in sexual desire could be demonstrated.
The advisory committee of the US Food and Drug Administration (FDA) did not see the efficacy of the study data as sufficiently proven and at the same time criticized the comparatively poor tolerability. The committee therefore recommended that flibanserin not be approved for the treatment of hypoactive sexual dysfunction and requested further evidence of efficacy and safety. In October 2010, Boehringer announced that it would stop developing Flibanserin for the time being. The US company Sprout Pharmaceuticals then took over the further development of the preparation, which was approved by the FDA for the US market in August 2015 . Immediately after approval, Sprout Pharmaceuticals was acquired by Canadian pharmaceutical company Valeant .
There is no drug approval in Europe .
Media reception
In connection with flibanserin, the media often spoke of “ Viagra for women” or “Female Viagra” in English. However, this comparison is not correct with regard to indication and effect.
Commercial preparations
Addyi (USA)
Individual evidence
- ↑ There is not yet a harmonized classification for this substance . A labeling of 1,3-dihydro-1- (2- (4- (3- (trifluoromethyl) phenyl) -1-piperazinyl) ethyl) -2H-benzimidazol-2-one in the classification is shown, which is derived from a self-classification by the distributor and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on March 27, 2020.
- ↑ F. Borsini, K. Evans, K. Jason, F. Rohde, B. Alexander, S. Pollentier: Pharmacology of flibanserin . In: CNS Drug Rev . tape 8 , no. 2 , 2002, p. 117-142 , PMID 12177684 .
- ↑ a b c d Division of Reproductive and Urologic Products Office of New Drugs Center for Drug Evaluation and Research, Food and Drug Administration: Background Document for Meeting of Advisory Committee for Reproductive Health Drugs (June 18, 2010). NDA 22-526. Flibanserin. (Proposed trade name: Girosa). Boehringer Ingelheim. (PDF; 3.9 MB) May 20, 2010, accessed on July 3, 2010 .
- ↑ M. Poplawska, A. Blazewicz, P. Zolek, Z. Fijalek: Determination of flibanserin and tadalafil in supplements for women sexual desire enhancement using high-performance liquid chromatography with tandem mass spectrometer, diode array detector and charged aerosol detector. In: J Pharm Biomed Anal. 94, Jun 2014, pp. 45-53. PMID 24531007
- ↑ D. Biermann: Flibanserin fails with FDA . In: Pharmaceutical newspaper . No. 26 , 2010 ( pharmische-zeitung.de ).
- ↑ Pharmaceutical company stops lust pill for women. In: Spiegel online. October 8, 2010.
- ↑ FDA approves first treatment for sexual desire disorder. FDA press release, August 18, 2015.
- ↑ Sprout Pharmaceuticals Receives FDA Approval of ADDYI ™ (Flibanserin 100 MG) ( Memento of the original from August 20, 2015 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. , Sprout Pharmaceuticals PM on August 18, 2015, accessed August 19, 2015.
- ↑ Takeover of Sprout Pharmaceuticals: Valeant swallows manufacturer of the pleasure pill. ( Memento from August 20, 2015 in the Internet Archive ) on: tagesschau.de , August 20, 2015.