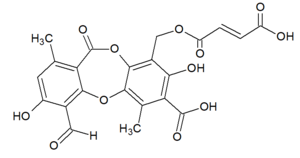
Fumarprotocetraric acid
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Fumarprotocetraric acid | ||||||||||||||||||
| other names |
[(7-Carboxy-4-formyl-3,8-dihydroxy-1,6-dimethyl-11-oxo-11 H -dibenzo [ b , e ] [1,4] dioxepin-9-yl) methyl] hydrogen fumarate ( IUPAC ) |
||||||||||||||||||
| Molecular formula | C 22 H 16 O 12 | ||||||||||||||||||
| Brief description |
colorless, odorless solid with a bitter taste |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 472.36 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
approx. 250 ° C (brown-black without melting) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Fumarprotocetraric acid is a colorless, odorless solid which, as a lichen substance, belongs to the group of depsidones. Typically for depsidone, the acid is a derivative of 11 H -dibenzo [ b , e ] [1,4] dioxepin-11-one. The latter compound consists of two phenol nuclei, which are linked to one another via a -CO-O bridge and an ether bond .
As lichen acid of the benzene series, fumarprotocetric acid is one of the derivatives of orcin (3,5-dihydroxytoluene) and is assigned to the protocetraric acid clan within the psoromic acid group.
Occurrence
The substance is best known for its occurrence in Icelandic moss ( Cetraria islandica [ L. ]). It is contained in a mass fraction of 1 - 2% (dried thallus).
In addition, fumarprotocetraric acid has been detected in the following lichens, among others :
- Callopisma teicholytum [ Ah. ]
- Cetraria fahluensis [ L. ]
- Cetraria islandica [ L. ]
- Cladina rangiferina [ L. ]
- Cladina silvatica [ L. ]
- Cladonia chlorophaea [ Flörke ]
- Cladonia fimbriata [ L. ] var. Apolepta [ Ach. ] f. coniocraea [ Flörke ]
- Cladonia fimbriata [ L. ] var. Cornuto-radiata [ Coem. ]
- Cladonia fimbriata [ L. ] var. Simplex [ Weis ] f. major [ Hag. ]
- Cladonia fimbriata [ L. ] var. Simplex [ Weis ] f. minor [ Hag. ]
- Cladonia foliacea [ Huds. ] var. alcicornis [ Lightf. ]
- Cladonia foliacea [ Huds. ] var. convoluta [ Lam. ]
- Cladonia furcata [ Huds. ] var. pinnata [ Flörke ]
- Cladonia furcata [ Huds. ] var. racemosa [ Hoffm. ]
- Cladonia gracilis [ L. ] var. Chordalis [ Flörke ]
- Cladonia gracilis [ L. ] var. Elongata [ Jacq. ]
- Cladonia pityrea [ Flörke ] var. Cladomorpha [ Flörke ]
- Cladonia pityrea [ Flörke ] var. Zwackhii [ Wainio ]
- Cladonia pyxidata [ L. ] var. Cereina [ Arnold ]
- Cladonia pyxidata [ L. ] var. Neglecta [ Flörke ]
- Cladonia subcervicornis [ Wainio ]
- Cladonia verticillata [ Hoffm. ] var. cervicornis [ Ach. ] f. phyllophora [( Flörke ) Sandstede ]
- Cladonia verticillata [ Hoffm. ] var. evoluta [ Wainio ]
- Dendrographa leucophaea [ Tuck. ]
- Parmelia stygia [( L. ) Ah. ]
Extraction and presentation
Fumarprotocetraric acid can be isolated from the powdered thallus of Cetraria islandica [ L. ]. Here, the plant material is extracted with acetone under protection from light until it is exhausted . The acid precipitates out of the strongly concentrated acetone extract when standing in the cold for a long time. The precipitate is filtered off with suction and washed on the filter with cold diethyl ether to remove chlorophyll until the acid is predominantly white. Pure fumarprotocetraric acid in the form of colorless needles can then be obtained by recrystallization from hot acetone.
properties
Physical Properties
The acid does not melt and therefore does not have a precisely determinable melting point; rather, decomposition occurs at higher temperatures. At approx. 240 ° C, the solid becomes brownish. From approx. 250 - 260 ° C the color turns black. At about 270 ° C, fumaric acid ( trans- butenedioic acid) sublimes , which precipitates in the form of relatively large, colorless crystals in the upper area of the melting point determination capillary. These decomposition properties can be used to identify the acid.
Fumarprotocetraric acid is colorless, odorless and tastes strongly bitter. The compound is very difficult to dissolve in hot water. The aqueous solution is acidic and tastes bitter. Chloroform , benzene and petroleum ether ( ligroin ) do not dissolve even when heated. Boiling diethyl ether is extremely difficult to dissolve, while absolute boiling ethanol and glacial acetic acid are very difficult to dissolve. Boiling acetone is difficult to dissolve, but it is best and most indifferent.
The UV-VIS spectrum of fumarprotocetraric acid (2 mg substance dissolved in 50.0 mL methanol ) provides two absorption maxima:
λ max1 = 238 - 239 nm (ε = 6092)
λ max2 = 315 - 316 nm (ε = 1150)
Chemical properties
Alkalis and their carbonates (sodium hydroxide solution, potassium hydroxide solution, sodium carbonate, potassium carbonate, potassium hydrogen carbonate) dissolve fumarprotocetraric acid easily and with a yellow color, which becomes darker over time. The acid breaks down into fumaric acid and protocetraric acid. When excess hydrochloric acid is added to this solution, the protocetraric acid and a small part of the fumaric acid precipitate. Most of the fumaric acid, however, remains in solution. If it is then extracted with diethyl ether, a large part of the fumarprotocetraric acid is formed back.
Concentrated sulfuric acid dissolves with red color. If you then add a lot of water, flakes fall out, which appear red to red-brown on the filter.
The ethanolic solution reacts acidic and turns purple with traces of iron (III) chloride.
When the acid is boiled with hydrochloric or sulfuric acid-containing ethanol, the solution turns blue with a blue-green precipitate.
If fumarprotocetraric acid is boiled with alkaline ethanol, cetraric acid and fumaric acid are formed. After hydrochloric acid has been added to the reaction liquid, the cetraric acid precipitates while the fumaric acid remains in solution. In this way, pure cetraric acid can ultimately be obtained by filtering off the precipitate and recrystallizing it from hot ethanol.
pharmacology
The substance is primarily said to have an antibacterial effect. It induces an immunostimulating effect in vitro by triggering the formation of hydrogen peroxide . Furthermore, cytotoxicity against two cancer cell lines could be demonstrated.
proof
In thin layer chromatography , fumarprotocetraric acid can be detected, for example, by spraying the plate with anisaldehyde-sulfuric acid reagent. After heating to approx. 110 ° C, the corresponding zone turns gray-blue. Another possibility of detection is spraying with a freshly prepared solution of 10 mg p- phenylenediamine in 10 mL ethanol. The fumarprotocetraric acid shows an ocher-yellow fluorescence in the UV 365 light. In general, p- phenylenediamine is a typical reagent for lichen acids.
literature
- Egon Stahl, Werner Schild: Isolation and characterization of natural substances . 1st edition. Gustav Fischer Verlag, Stuttgart / New York 1986.
- Wilhelm Zopf: The lichen substances in chemical, botanical, pharmacological and technical relationship . 1st edition. Gustav Fischer Verlag, Jena 1907.
- Emil Abderhalden (Ed.): Biochemisches Handlexikon . Tannins, lichen substances, saponins, bitter substances, terpenes, essential oils, resins, rubber . 1st edition. Springer Verlag, Berlin / Heidelberg 1912.
Individual evidence
- ↑ a b c d e f g Entry on fumarprotocetraric acid. In: Römpp Online . Georg Thieme Verlag, accessed on July 28, 2018.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Entry on Depsidone. In: Römpp Online . Georg Thieme Verlag, accessed on July 28, 2018.
- ↑ a b c d e f g h i j k l m n o p q r s t u Wilhelm Zopf: The lichen substances in chemical, botanical, pharmacological and technical relation . 1st edition. Gustav Fischer Verlag, Jena 1907, p. 172-176 .
- ↑ a b c d e f Egon Stahl, Werner Schild: Isolation and characterization of natural substances . 1st edition. Gustav Fischer Verlag, Stuttgart / New York 1986, p. 92 ff .
- ↑ a b c d e f g h i j k l m n o p q r s t u v w x Emil Abderhalden (Ed.): Biochemisches Handlexikon. Tannins, lichen substances, saponins, bitter substances, terpenes, essential oils, resins, rubber . 1st edition. Springer Verlag, Berlin / Heidelberg 1912, p. 74 f .
