Furilazole
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| 1: 1 mixture of ( S ) -form (top) and ( R ) -form (bottom) |
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Furilazole | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 11 H 13 Cl 2 NO 3 | ||||||||||||||||||
| Brief description |
light yellow powder |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 278.13 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
96.6-97.6 ° C |
||||||||||||||||||
| solubility |
poorly soluble in water (0.197 g l −1 at 20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Furilazole is a plant protection agents from the group of 1,3-oxazolidines .
Extraction and presentation
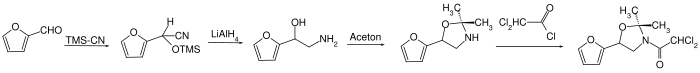
Furilazole can be obtained by a multi-stage reaction of 2-furaldehyde with cyanotrimethylsilane , lithium aluminum hydride , acetone and dichloroacetyl chloride.
properties
Furilazole is a yellowish solid that is poorly soluble in water.
use
Furilazole is used as a herbicide safener . It was developed by Monsanto in the 1990s and marketed as an additive for halosulfuron-methyl from 1995 .
In Germany, Austria and Switzerland, no pesticides with this active ingredient are permitted.
Individual evidence
- ↑ a b c d e f g h i data sheet Furilazole from Sigma-Aldrich , accessed on May 21, 2017 ( PDF ).
- ↑ Thomas A. Unger: Pesticide Synthesis Handbook . William Andrew, 1996, ISBN 0-8155-1401-8 , pp. 490 ( limited preview in Google Book search).
- ↑ Wolfgang Krämer, Ulrich Schirmer, Peter Jeschke, Matthias Witschel: Modern Crop Protection Compounds . John Wiley & Sons, 2012, ISBN 978-3-527-32965-6 , pp. 378 ( limited preview in Google Book search).
- ^ Directorate-General for Health and Food Safety of the European Commission: EU pesticide database ; Entry in the national registers of plant protection products in Switzerland , Austria and Germany ; accessed on March 12, 2016.