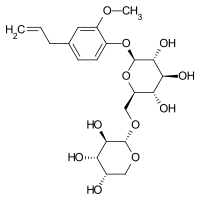
Gein (chemical compound)
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||
| General | ||||||||||
| Surname | Ok | |||||||||
| other names |
|
|||||||||
| Molecular formula | C 21 H 30 O 11 | |||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 458.46 g · mol -1 | |||||||||
| Melting point |
146-147 ° C |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Gein (pronunciation: Ge-in , synonym: geoside ) is a chemical compound . It is a glycoside of eugenol .
Occurrence
Gein could be isolated from the common carnation root ( Geum urbanum ). It occurs in all parts of the plant. Gein is believed to be a by-product of lignin biosynthesis .
Structure and properties
Gein represents the vicianoside (glycoside with glucose and arabinose ) of eugenol. Gein is split into eugenol and the disaccharide vicianose by the enzyme gease . For this reason, steam distillation produces an essential oil , which mainly consists of eugenol, after brief maceration of crushed root parts . The specific angle of rotation of an aqueous solution of Gein is −53 ° to −54.4 °.
Individual evidence
- ↑ a b c Walter Karrer: Constitution and occurrence of organic plant substances. Springer-Verlag, 1958, ISBN 978-3-034-86808-2 , p. 81.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ R. Hegnauer: About the glycoside Gein (= geoside) in the genus Geum . In: Phyton , 5, 1954, pp. 194-203, PDF .
- ↑ a b R. Hegnauer: Chemotaxonomie der Pflanzen, Volume 6. Springer-Verlag, 1973, ISBN 978-3-034-89379-4 , p. 108.
- ↑ YPS Bajaj: Medicinal and Aromatic Plants V. Springer Science & Business Media, 2012, ISBN 978-3-642-58062-8 , p. 134.
- ↑ Rudolf Hänsel: Hager's Handbook of Pharmaceutical Practice, Volume 5. Springer-Verlag, 1993, ISBN 978-3-642-57993-6 , p. 260.
