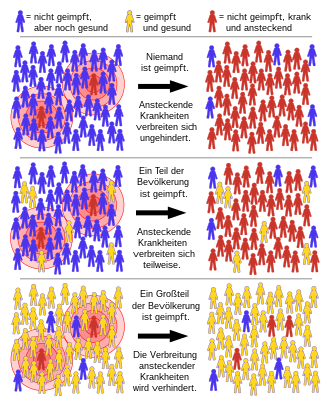
Herd immunity
Herd immunity (from English herd immunity ) referred to in the epidemiology an indirect form of protection against infectious disease , which occurs when a high percentage of the population has already become immune - either through infection or by vaccination - is so spread capabilities of the pathogen decrease within the population as a whole. This indirectly results in increased protection for non-immune individuals as well. The term must be clearly distinguished from that of individual immunity .
Definitions
Herd immunity can be understood as:
- the proportion of a population (the “herd”) that is immune to certain communicable infectious diseases
- a parameter for the proportion of immunized persons in a population which, if exceeded, leads to a decrease in new infections in this population
- a pattern of immunity that protects a population from new infections
- the resistance of a group to attack by a disease
- the phenomenon that not every person in a population needs to be immunized to eradicate a disease
Moreover, the term herd immunity is in different ways with the term "herd effect" (English of. Herd effect ) associated:
- Herd effect is the indirect protection that is granted to the non-immunized part of the population, provided that those protected by infection or vaccination interrupt the chain of infection (also called community protection )
- Herd immunity is synonymous with the herd effect
The term herd immunity is thus used with different meanings. An understanding of the immunological phenomena associated with comprehensive immunization coverage of populations would help to improve the design of vaccination campaigns so that communicable infectious diseases can be better controlled, limited or eradicated not only in theory but also in reality. The definition of herd immunity as direct and herd effect as indirect protection is followed below.
Influencing variables
The phenomenon of herd immunity is complex. The simple (“crude”) threshold theory, ie the basic model for the interaction of transmission and immunity threshold, is “naive” because it is based on a number of assumptions that are not in reality and on factors that have not yet been established are sufficiently understood or quantifiable or still unknown.
The variables that have an impact on the actually achieved effectiveness (efficiency) of vaccinations include:
- Maternal immunity, with its effect on neonatal loan immunity and the immunogenicity of newborn vaccinations
- Age especially at the first vaccination
- Age with an effect on the type and frequency of contact with germ carriers
- Season with an effect on the type and frequency of contact with germ carriers and the ability of germs to multiply
- Duration of infectivity
- Duration and level of immunity
- Homogeneity of the population or of sub-populations in terms of type and frequency of contact and the transmission of infection between infected and non-immunized
- Homogeneity of the population or of sub-populations with regard to attitudes towards and compliant implementation of vaccinations
- Distribution of vaccines (random or targeted, homogeneous or inhomogeneous)
- Contact of sub-populations to the rest of the population (isolated or permeable)
- Distribution of immunity in sub-populations and the population
- individually impaired immune competence (for example due to immunodeficiency or vaccination failure )
- collective vaccination failure in sub-populations (e.g. due to hygienic conditions, nutritional status, ethnic-genetic differences)
- Type of immunity induced by vaccination (e.g. humoral or cellular, mucosal or systemic, oral or enteral, against the pathogen or against toxins)
The efficiency factor E introduced into the mathematical model to take these influencing variables into account is in turn based on assumptions as to how the aforementioned influencing variables affect quantitatively individually and in mutual interaction.
As a rule, factor E leads to an increase in the vaccination rate required in reality compared to the idealistic assumptions of the mathematical model. If, however, sub-populations are known that have a particularly high risk of infection, their targeted vaccination can lower the overall vaccine coverage required, i.e. lead to an average higher actual effectiveness of the vaccine campaign.
properties
The herd effect works like a firebreak in a fire, in that the chain of infection of a pathogen is interrupted or at least slowed down with vaccinations. As a result, the disease can no longer spread epidemically if the pathogens are only transmitted between people ( anthroponosis ). Herd immunity cannot be achieved against pathogens that survive outside their host, for example the bacterium Clostridium tetani , which occurs in the soil, which triggers tetanus or the TBE virus transmitted by ticks . Protection here is only offered by individual prophylaxis.
Above a certain threshold of immunity in a population, the population of the pathogen collapses due to a lack of reproduction and the disease can no longer circulate in this population. This threshold value is essentially dependent on the basic reproduction number of the respective pathogen. If the pathogen has refuge areas in other populations, it can no longer infect from these refuge areas from populations with sufficient herd immunity.
| illness | Transmission path | R 0 | Minimum proportion of immunized |
|---|---|---|---|
| measles | Droplet infection | 12-18 | 83-94% |
| mumps | Droplet infection | 4-7 | 75-86% |
| polio | fecal-oral infection | 5-7 | 80-86% |
| rubella | Droplet infection | 5-7 | 80-85% |
| smallpox | Droplet infection | 6-7 | 83-85% |
|
The base reproduction number R 0 indicates how many other people are infected on average by an infected person if the population or subpopulation surrounding them is neither protected by vaccination nor by previous infection. |
|||
The basic model
The minimum vaccination coverage required for a herd effect under ideal circumstances is given by:
and in percentages:
- .
Here is is the basic reproduction number. The value is also called herd immunity threshold ( english herd immunity threshold , short- HIT ), respectively.
The herd immunity threshold (HIT) listed in the table is not identical to the minimum required vaccination rate:
Efficiency factor E
In contrast to the basic model, the extent of herd immunity and herd effect that can actually be achieved depends on the aforementioned influencing factors. Therefore, in reality, higher vaccination rates are usually required for a herd effect than under ideal conditions. To take this into account, the equation is expanded to include the quantity E, the factor of effectiveness ( for efficiency):
Thus, the minimum vaccination coverage required is usually higher than the minimum required herd immunity (HIT) listed in the table. Both an increase in the coverage rate and an increase in the effectiveness of the vaccination make it easier to achieve a herd effect. The favorable case in which the minimum necessary vaccination rate required on average (for a total population) is lower than the minimum necessary herd immunity listed in the table (for the total population) rarely occurs, for example in the case of targeted vaccination of sub-populations with a particularly high risk infection and / or transmission. Herd immunity is not a static phenomenon. For example, vaccinations with waning immunity over time require booster vaccinations to maintain a herd effect.
Protection of special groups of people
People who cannot be vaccinated effectively
Herd immunity is of particular importance for people who cannot be vaccinated with sufficient effectiveness, for example people with immunosuppression (disease of the immune system such as HIV infection, lymphoma , bone marrow cancer or leukemia ; chemotherapy or radiation therapy ; taking immunosuppressants after organ transplantation). In addition, contraindications can contribute to a lack of immunity. The lack of immunity in these groups of people can sometimes also lead to more severe disease processes, which can be avoided by a herd effect.
People who cannot be vaccinated yet
For example, side effects (especially from vaccines with live pathogens) or ineffectiveness due to passive immunity from the mother's antibodies can be reasons for administering individual vaccines in newborns . Pregnant women who are not yet protected from rubella by infection or vaccination are not vaccinated against rubella with the currently common combination vaccines because they contain live pathogens whose harmlessness for the unborn is not guaranteed. The herd immunity of their contact persons serves to protect such pregnant women and their unborn babies.
Protection of other age groups
High vaccination coverage in one age group can also protect people in other age groups from developing this pathogen, such as unvaccinated infants and young children or the elderly.
Sexually transmitted diseases
High-risk behavior during sexual intercourse leads to a high transmission rate of the pathogens concerned, which makes the eradication of sexually transmitted diseases more difficult. With sexually transmitted diseases , the herd effect may extend from one sex to the other sex.
Eradication

In the best case scenario, a disease can even be eradicated ( eradication ) by means of sufficiently high vaccination rates . In other words, the pathogen is no longer endemic. The eradication of infectious diseases is a goal of health policy . In the case of smallpox , this was achieved through a consistent, worldwide vaccination and control program, so that in 1980 the WHO declared the world to be free of smallpox . The same has now almost been achieved for polio globally - only a few countries are still considered endemic for polio viruses ( Nigeria , India , Pakistan , Afghanistan ). However, as vaccination efforts decline in neighboring countries, there are repeated outbreaks of poliomyelitis through re-imports, most recently in 2006 in Namibia . The global elimination of measles, also set as a goal by the WHO, has so far only been achieved on the continents of America and Australia as well as in Scandinavia, as the vaccination rates are too low in the rest of the world. As a result, local measles epidemics break out again and again, including in Germany, for example, the regionally limited measles epidemics in Hesse, Bavaria, Baden-Württemberg and North Rhine-Westphalia, including serious complications and deaths in 2005/2006.
Negative epidemiological effects
As a rule, high vaccination coverage results in herd immunity and a herd effect. Large populations around the world have been protected from dangerous communicable infectious diseases. However, the findings of the pharmacologist Gustav Kuschinsky also apply to vaccines : "A drug that is claimed to have no side effects is strongly suspected of not having any main effect." According to the WHO, side effects of vaccinations should be specifically recorded and systematically classified become. In addition to individual side effects for some vaccinated persons (e.g. pain at the injection site, allergy to components of the vaccine), side effects of an epidemiological nature are also known. The Robert Koch Institute describes them as "undesirable negative effects of a vaccination strategy on the population level" and has declared their education to be one of its tasks. Examples of the negative epidemiological effects of vaccination at the population level include:
- Changes to prevailing serotypes (serotype replacement). The consequence can be, for example, that the effectiveness of previous vaccines decreases.
- Age shifts in the burden of disease. This can, for example, increase the likelihood and / or severity of complications from the infectious disease.
- Reverse mutation of attenuated live vaccines. It can lead to the formation of human pathogens in the vaccinated person and to the infection of third parties by these pathogens.
- Changes in predominant pathogen species. The type of pathogen that has become more common can, for example, have other living beings (“hosts”) as reservoirs in addition to humans and thus evade eradication.
Serotype replacement
Vaccinations can lead to a change in the relative and / or absolute frequency of the serotypes of the pathogen (vaccine-induced pathogen strain replacement). As far as the increase concerns serotypes that are not covered by the vaccine, the effect of the vaccination may be less than was to be expected based on the vaccine's effectiveness against the serotypes contained in the vaccine. A serotype replacement requires the replacement or expansion of the antigens in the vaccine. A major example of this is the pneumococcal vaccine .
Age shifts in the burden of disease
The herd effect can lead to an accumulation of people in sub-populations who are themselves immune to the pathogens of communicable infectious diseases, neither through infection nor vaccination. If germ carriers get into this group, for example through travel to endemic areas or immigration from such areas, and the herding effect is insufficient for this group, they risk developing an illness that was previously common as a childhood disease . Such age shifts have been documented for measles , for example . As people get older, measles is more difficult to detect, so that measles pneumonia , for example, is delayed and treated appropriately. In addition, measles occurs in newborns in the period up to the first vaccination (recommended between 11 and 14 months of age) when their mothers have been vaccinated against this pathogen than when the mothers had the measles as an infection because the loan immunity brought about via the placenta disappears faster after vaccination than after infection.
When it comes to mumps , the majority of infections in children run with little or no symptoms. In recent years, however, in Germany, as in many other European countries, more and more mumps outbreaks have occurred among adolescents and young adults. At this older age, the symptoms become more obvious, and in male patients they include the risk of sterility due to mumps orchitis .
With rubella , too, complications become more common as the sick person gets older. Also chickenpox and hepatitis A occur thanks of regulated vaccinations less frequently in childhood. If the herd effect diminishes, however, people who have not been vaccinated are often only infected at an advanced age. Then on average there are more frequent and more serious complications than in children.
After vaccination against whooping cough , antibodies pass through the placenta to the newborns. The pertussis antibodies, on the one hand, protect the newborns from life-threatening illnesses in the first two months and, on the other hand, (unlike occasional loan antibodies against rubella) do not hinder the development of their own antibodies in response to the newborn vaccinations that start in accordance with the rules from the end of the second month.
Reverse mutation of attenuated live vaccines
The most important example of this type of negative effects of population vaccinations is the reverse mutation of the pathogen in the oral polio vaccine (OPV) into a variant that is pathogenic to humans again , which is similar to the wild virus, and third party diseases caused by this virus ( circulating vaccine-derived poliovirus , cVDPV). The WHO therefore recommends that the “oral vaccination” with OPV be gradually switched over to the inactivated polio vaccine (IPV).
Increase in other pathogen species
As a result of the DTP vaccinations, the majority of reported diphtheria cases in Western Europe are caused by Corynebacterium ulcerans (skin diphtheria) and no longer by the previously classic diphtheria pathogen C. diphtheriae (pharynx diphtheria ). C. ulcerans is able to produce the diphtheria toxin and thus trigger the systemic symptoms of the disease. The usual diphtheria vaccine is also effective against C. ulcerans . But unlike C. diphtheriae, C. ulcerans has its reservoir in animals (including domestic animals), which makes its eradication more difficult.
Problems
Vaccination fatigue
Vaccination fatigue is a particular danger to herd immunity . Vaccination campaigns which do not achieve the necessary herd immunity can, under certain circumstances, increase the frequency of disease complications in non-vaccinated persons. If too low a proportion of the population is vaccinated, this “only” lowers the likelihood of infection among those who have not been vaccinated instead of preventing infection via herd immunity. This means that the infection, if it then occurs, often no longer occurs in childhood, which is more dangerous with some diseases such as mumps , rubella , polio , chickenpox . For example, an increase in rubella embryo fetopathy cases was reported in Greece in the early 1990s , after vaccination coverage was below 50% throughout the 1980s. For this reason, every vaccination campaign should not only aim to partially protect the population, but also ensure herd immunity. It is also important that those responsible for planning vaccination campaigns understand mathematical and epidemiological models of medicine. Education about the herd effect can reduce vaccination fatigue. In polio, eradication is delayed by political unrest and mistrust of modern medicine. A vaccination requirement could accelerate the eradication.
COVID-19 pandemic
Herd immunity in the course of the COVID-19 pandemic (caused by SARS-CoV-2 ) was mentioned and sometimes criticized in English-language and, occasionally, also in German media .
The British government under Boris Johnson initially advocated an - albeit uncontrolled (even without vaccination, which is / was not yet available) - herd immunity strategy to combat COVID-19 in the UK . But after warnings from science, she stopped doing it.
In an interview with Radio Eins , the German virologist Karin Mölling also suggested such an uncontrolled strategy. In an interview with KenFM on March 24, 2020, she said no when asked by Ken Jebsen whether she supported herd immunization. This is "today an ethically very dubious matter, because you basically let people run into the knife". It belongs to the "helplessness reactions because you have to try to do what", but "decision makers" should not "let people run into open knives". In an interview on April 8, 2020, forensic doctor Klaus Püschel considered a course of herd immunity to be inevitable.
In April 2020, it was still unclear in the ranks of the World Health Organization (WHO) whether herd immunity could even be developed with COVID-19. A complete immunity of former patients against a new infection lasting several months has not yet been adequately investigated, although animal studies and observed antibodies had suggested this. In South Korea, the virus was detected again using PCR in 91 patients who had already recovered; however, it remained unclear whether this corresponds to being sick again. This may have had various causes, including the always existing uncertainty with medical test results, including the detection methods of a Covid-19 disease . In another preliminary study from Shanghai, not all patients had developed the same high antibody levels, and in some the antibodies had not been detected in the laboratory.
Web links
- Herd immunity animation and interactive simulator. impfen-info.de
- Further information on the topic of the Scientific Academy for Preventive Medicine Graz
- How to achieve herd immunity dlf.de
Individual evidence
- ^ A b c d e f g Paul EM Fine, K. Eames, DL Heymann: “Herd immunity”: a rough guide. In: Clinical Infectious Diseases . Volume 52, Number 7, April 2011, pp. 911-916, doi: 10.1093 / cid / cir007 , PMID 21427399 .
- ↑ L. Gordis: Epidemiology . Elsevier Health Sciences, November 14, 2013, ISBN 978-1455742516 , pp. 26-27 (accessed March 29, 2015).
- ↑ a b c John T. Jacob, Samuel Reuben: Herd immunity and herd effect: new insights and definitions . In: Eur J Epidemiol. , 2000, 16 (7), pp. 601-606, doi: 10.1023 / a: 1007626510002 , JSTOR 3582376
- ↑ a b c d Paul EM Fine u. a .: Community Protection . In: Stanley A. Plotkin et al. a .: Vaccines . 7th edition. 2017, ISBN 978-0-323-35761-6 .
- ↑ a b c d e T. H. Kim, J. Johnstone, M. Loeb: Vaccine herd effect. In: Scandinavian journal of infectious diseases. Volume 43, number 9, September 2011, pp. 683-689, doi: 10.3109 / 00365548.2011.582247 , PMID 21604922 , PMC 3171704 (free full text).
- ^ JP Fox: Herd Immunity and Measles . In: Rev Infect Dis. , 1983 May-Jun, 5 (3), pp. 463-466, PMID 6879000
- ↑ a b E.M. Paul: Fine: Herd Immunity - History, Theory, Practice . In: Epidemiol Rev. , July 29, 1993, 15, pp. 265-302, PMID 8174658 , doi: 10.1093 / oxfordjournals.epirev.a036121
- ^ A b History and Epidemiology of Global Smallpox Eradication . ( Memento of the original from July 15, 2007 in the Internet Archive ; PDF; 1.5 MB) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. From the training course Smallpox: Disease, Prevention, and Intervention . CDC and WHO . Slide 16-17.
- ^ Gregg N. Milligan, Alan DT Barrett: Vaccinology. Wiley, 2015, ISBN 978-1-118-63628-2 , p. 313.
- ↑ A. McGirr, DN Fisman: Duration of pertussis immunity after DTaP immunization: a meta-analysis. In: Pediatrics. Volume 135, number 2, February 2015, pp. 331–343, doi: 10.1542 / peds.2014-1729 , PMID 25560446 .
- ↑ a b c d e S. Cesaro, M. Giacchino, F. Fioredda, A. Barone, L. Battisti, S. Bezzio, S. Frenos, R. De Santis, S. Livadiotti, S. Marinello, AG Zanazzo, D. Caselli: Guidelines on vaccinations in pediatric haematology and oncology patients. In: BioMed research international. Volume 2014, 2014, p. 707691, doi: 10.1155 / 2014/707691 , PMID 24868544 , PMC 4020520 (free full text)
- ↑ RM Wolfe: Update on adult immunizations. In: Journal of the American Board of Family Medicine: JABFM. Volume 25, number 4, 2012 Jul-Aug, pp. 496-510, doi: 10.3122 / jabfm.2012.04.100274 , PMID 22773718
- ^ S. Esposito, S. Bosis, L. Morlacchi, E. Baggi, C. Sabatini, N. Principi: Can infants be protected by means of maternal vaccination? In: Clinical microbiology and infection: the official publication of the European Society of Clinical Microbiology and Infectious Diseases. Volume 18 Suppl 5, October 2012, pp. 85-92, doi: 10.1111 / j.1469-0691.2012.03936.x , PMID 22862749
- ↑ a b c d D. Rakel, RE Rakel: Textbook of Family Medicine . Elsevier Health Sciences, 2015, ISBN 978-0-323-31308-7 , pp. 99, 187 (accessed March 30, 2015).
- ↑ a b T. H. Tulchinsky, EA Varavikova: The New Public Health: An Introduction for the 21st Century . Academic Press, March 26, 2014, ISBN 978-0-12-415767-5 , pp. 163-82 (accessed March 30, 2015).
- ↑ FM Munoz: Maternal immunization: an update for pediatricians. In: Pediatric annals. Volume 42, Number 8, August 2013, pp. 153-158, doi: 10.3928 / 00904481-20130723-09 , PMID 23910028 .
- ↑ General recommendations on immunization - recommendations of the Advisory Committee on Immunization Practices (ACIP). In: MMWR. Recommendations and reports: Morbidity and mortality weekly report. Recommendations and reports. Volume 60, Number 2, January 2011, pp. 1-64, PMID 21293327 .
- ↑ LF Pittet, KM Posfay-Barbe: Pneumococcal vaccines for children: A global public health priority . In: Clinical Microbiology and Infection . 18 Suppl 5, 2012, pp. 25-36. doi : 10.1111 / j.1469-0691.2012.03938.x . PMID 22862432 .
- ↑ O Nakagomi, M Iturriza-Gomara, T Nakagomi, NA Cunliffe: Incorporation of a rotavirus vaccine into the national immunization schedule in the United Kingdom: A review . In: Expert Opinion on Biological Therapy . 13, No. 11, 2013, pp. 1613-1621. doi : 10.1517 / 14712598.2013.840285 . PMID 24088009 .
- ↑ BA Lopman, DC Payne, JE Tate, MM Patel, MM Cortese, UD Parashar: Post-licensure experience with rotavirus vaccination in high and middle income countries; 2006 to 2011 . In: Current Opinion in Virology . 2, No. 4, 2012, pp. 434-442. doi : 10.1016 / j.coviro.2012.05.002 . PMID 22749491 .
- ↑ TH Kim: Seasonal influenza and vaccine herd effect . In: Clinical and Experimental Vaccine Research . 3, No. 2, 2014, pp. 128–32. doi : 10.7774 / cevr.2014.3.2.128 . PMID 25003085 . PMC 4083064 (free full text).
- ↑ a b c G. P. Garnett: Role of herd immunity in determining the effect of vaccines against sexually transmitted disease. In: The Journal of Infectious Diseases . Volume 191 Suppl 1, February 2005, pp. S97-106, doi: 10.1086 / 425271 , PMID 15627236 .
- ↑ a b c A Lenzi, V Mirone, V Gentile, R Bartoletti, V Ficarra, C Foresta, L Mariani, S Mazzoli, SG Parisi, A Perino, M Picardo, CM Zotti: Rome Consensus Conference - statement; human papilloma virus diseases in males . In: BMC Public Health . 13, 2013, p. 117. doi : 10.1186 / 1471-2458-13-117 . PMID 23391351 . PMC 3642007 (free full text).
- ^ DR Lowy, JT Schiller: Reducing HPV-associated cancer globally . In: Cancer Prevention Research . 5, No. 1, 2012, pp. 18-23. doi : 10.1158 / 1940-6207.CAPR-11-0542 . PMID 22219162 . PMC 3285475 (free full text).
- ^ SM Garland, SR Skinner, JM Brotherton: Adolescent and young adult HPV vaccination in Australia: Achievements and challenges . In: Preventive Medicine . 53 Suppl 1, 2011, pp. 29-35. doi : 10.1016 / j.ypmed.2011.08.015 . PMID 21962468 .
- ↑ F. Njeumi, W. Taylor, A. Diallo, K. Miyagishima, PP Pastoret, B. Vallat, M. Traore: The long journey: a letter review of the eradication of rinderpest. In: Revue scientifique et technique. Volume 31, number 3, December 2012, pp. 729-746, doi: 10.20506 / rst.31.3.2157 , PMID 23520729 .
- ^ F. Fenner: The global eradication of smallpox. In: Med J Aust. , 1980 May 17,1 (10), p. 455. PMID 7412674
- ↑ Polio situation worldwide in 2008 - update on the progress towards global eradication . In: Eurosurveillance , 2009 Apr 16; 14 (15), pii: 19178. PMID 19371512
- ^ Poliomyelitis in Namibia . In: WHO - Disease Outbreak News , June 6, 2006
- ↑ Measles in 2005 and outbreaks in Baden-Württemberg and North Rhine-Westphalia in the first half of 2006 . (PDF) Robert Koch Institute : Epidemiological Bulletin , No. 27, July 7, 2006.
- ↑ No drug without side effects . Edited by the Association of Researcher Drug Manufacturers. V., online February 1, 2018, accessed October 10, 2019
- ^ Adverse Events Following Immunization (AEFI): Causality Assessment . (PDF) WHO , 2005, RHO Cervical Cancer - rho.org; accessed October 10, 2019
- ↑ a b Methods for the implementation and consideration of modeling for the prediction of epidemiological and health-economic effects of vaccinations for the Standing Vaccination Commission: Section 1.2.9: Indirect vaccination effects . (PDF) Robert Koch Institute , as of March 16, 2016
- ↑ Maia Martcheva et al. a .: Vaccine-induced pathogen strain replacement: what are the mechanisms? In: JR Soc Interface , 2008 Jan 6, 5 (18), pp. 3–13, doi: 10.1098 / rsif.2007.0236 , PMID 17459810 , PMC 2405901 (free full text)
- ↑ a b Scientific justification for changing the pneumococcal vaccination recommendation for infants . (PDF) Communication from the Standing Vaccination Commission (STIKO) at the RKI, September 7, 2015
- ↑ Vaccination Plan Austria 2019, Version 1. (PDF) Ministry of Social Affairs of the Republic of Austria, as of January 2019; accessed October 18, 2019
- ↑ a b c Manuel Battegay: Hearth immunity: Protect your neighbor - vaccinate yourself . Lecture at the Cantonal Hospital St.Gallen, May 2003, infekt.ch (PDF)
- ^ Pink Book - Measles . Centers for Disease Control and Prevention , April 15, 2019, accessed October 10, 2019, cdc.gov (PDF)
- ↑ Changed vaccination recommendation for mumps . (PDF) In: Epidemiological Bulletin , August 6, 2012 / No. 31, Robert Koch Institute
- ↑ RKI advice rubella , as of March 26, 2018
- ↑ Centers for Disease Control and Prevention: Pregnancy and Whooping Cough , September 28, 2017, accessed October 10, 2019
- ↑ Romesa Ibrahim et al. a .: Impact of maternally derived pertussis antibody titers on infant whole-cell pertussis vaccine response in a low income setting . In: Vaccine , 2018 Nov 12; 36 (46), pp. 7048–7053, doi: 10.1016 / j.vaccine.2018.09.045 , PMID 30297122 , PMC 6219892 (free full text)
- ↑ Diphtheria: disease caused by toxigenic Corynebacterium ulcerans after contact with cats - case report . (PDF) In: Epidemiologisches Bulletin , Robert Koch Institute , 2011 (27), pp. 245–248.
- ↑ Cutaneous diphtheria: Increase in infections with Corynebacterium ulcerans. Deutsches Ärzteblatt , March 15, 2018, accessed November 4, 2019 .
- ↑ Lauren M. Weil: Notes from the Field: Conjunctivitis Caused by Toxigenic Corynebacterium ulcerans - Missouri, 2018 . In: WHO Morbidity and Mortality Weekly Report (MMWR) , 68 (27), July 12, 2019, pp. 615–616.
- ↑ Takis Panagiotopoulos, Ioanna Antoniadou, Eleni Valassi-Adam: Increase in congenital rubella occurrence after immunization in Greece: retrospective survey and systematic review . In: BMJ , 319, 1999, pp. 1462-1467.
- ^ W. John Edmunds: Health professionals do not understand mathematical models . In: BMJ , 320, 2000, p. 581
- ↑ J. Logan, D. Nederhoff, B. Koch, B. Griffith, J. Wolfson, FA Awan, NE Basta: What have you HEARD about the HERD? Does education about local influenza vaccination coverage and herd immunity affect willingness to vaccinate? In: Vaccine . Volume 36, number 28, 06 2018, pp. 4118-4125, doi: 10.1016 / j.vaccine.2018.05.037 , PMID 29789242 , PMC 6008254 (free full text).
- ^ KA Smith: Smallpox: can we still learn from the journey to eradication? In: The Indian journal of medical research. Volume 137, Number 5, May 2013, pp. 895-899, PMID 23760373 , PMC 3734679 (free full text).
- ↑ A Perisic, CT Bauch: Social contact networks and disease eradicability under voluntary vaccination . In: PLoS Computational Biology . 5, No. 2, 2009, p. E1000280. doi : 10.1371 / journal.pcbi.1000280 . PMID 19197342 . PMC 2625434 (free full text).
- ^ F Fu, DI Rosenbloom, L Wang, MA Nowak: Imitation dynamics of vaccination behavior on social networks . In: Proceedings of the Royal Society B: Biological Sciences . 278, No. 1702, 2011, pp. 42-49. doi : 10.1098 / rspb.2010.1107 . PMID 20667876 . PMC 2992723 (free full text).
- ^ S Wicker, HC Maltezou: Vaccine-preventable diseases in Europe: Where do we stand? . In: Expert Review of Vaccines . 13, No. 8, 2014, pp. 979-87. doi : 10.1586 / 14760584.2014.933077 . PMID 24958075 .
- ^ E. Fukuda, J. Tanimoto: Impact of Stubborn Individuals on a Spread of Infectious Disease under Voluntary Vaccination Policy . Springer, 2014, ISBN 978-3-319-13359-1 , pp. 1–10 (accessed on March 30, 2015).
- ↑ telegraph.co.uk: What is herd immunity and will it stop coronavirus in the UK? , March 16, 2020, accessed March 16, 2020.
- ↑ washingtonpost.com: UK resists coronavirus lockdowns, goes its own way on response , March 16, 2020, accessed March 16, 2020.
- ↑ tagesspiegel.de: Johnson waives the hard way , March 15, 2020, accessed March 16, 2020.
- ↑ Medicine: Virologist Mölling warns of scare tactics , with clarification by the editors, Radio Eins , March 14, 2020
- ↑ The corona crisis - are the measures sufficient? , Phoenix Round, Phoenix , March 17th, 2020.
- ^ A b On corona virology: Prof. Karin Mölling on the phone. Telephone interview with Karin Mölling on KenFM , March 24, 2020, accessed on May 4, 2020.
- ↑ Coronavirus forensics: "We cannot prevent infections". Interview with forensic doctor Klaus Püschel on n-tv Wissen, April 8, 2020, accessed on May 4, 2020.
- ↑ After new cases in South Korea: Are you really immune after a corona infection? The WHO doubts this. In: Der Tagesspiegel , April 18, 2020, accessed on May 4, 2020.
- ↑ Coronavirus Survivors Hope for Immunity - The Reality Is More Complicated (English), bloomberg.com, April 14, 2020.