Heterobenzenes
Heterobenzenes are heteroaromatics with the empirical formula
- C 5 H 5 E (E = N, P, As, Sb, Bi) or
- C 5 H 6 E (E = Si, Ge, Sn)
The former are derived from benzene (C 6 H 6 ) by replacing a methine group (CH) with an ( isoelectronic ) heteroatom of the 15th group of the periodic table (PSE). The latter contain - like benzene - six hydrogen atoms but one carbon atom less, this has been replaced by another element from the 14th group of the PSE.
properties
All heterobenzenes have in common their aromaticity . The stability decreases with increasing atomic number of the heteroatom . The bond lengths and angles of the heterobenzenes of group 15 of the periodic table are shown below (from left to right: pyridine , phosphabenzene , arsabenzene , stibabenzene and bismabenzene ):
In 1982 Gottfried Märkl provided a compilation of the properties, syntheses and reactions of the pyridine homologues phosphabenzene and arsabenzene .
Silabenzol , Germabenzol and Stannabenzol are aromatic heterobenzenes that each contain a heteroatom (Si, Ge or Sn) from the 14th group of the PSE in the six-membered ring.
Occurrence and synthesis
Occurrence
The extraction of pyridine from coal tar, which contains about 0.1% of it, is of historical importance. Few natural occurrences of free pyridine are known. However, it could be detected in the volatile components of the marshmallow as well as the leaves and roots of the black belladonna ( Atropa belladonna ). Nothing is known about natural occurrences of phosphabenzene, arsabenzene, stibabenzene and bismabenzene as well as silabenzene, germabenzene and stannabenzene.
synthesis
Modern industrial syntheses of pyridine use the route published by Tschitschibabin for the first time in 1924, which is a multicomponent reaction between ketones or aldehydes with ammonia .
Phosphabenzene, arsabenzene, stibabenzene and bismabenzene are not manufactured on an industrial scale. However, they have considerable academic interest.
The transfer of Dilthey's pyridine synthesis from pyrilium salts and ammonia was also successful - under varying reaction conditions - for the preparation of 2,4,6-trisubstituted phosphabenzenes from hydrogen phosphide (PH 3 ).
The first synthesis of a substituted arsabenzene - more precisely 9-arsaanthracene - was described by Peter Jutzi and Friedrich Bickelhaupt .
A synthesis for the basic structure of several heterobenzenes was described by Arthur J. Ashe III, starting from trihalides (X = Cl, Br) of the elements of the 15th group of the periodic table (E = P, As, Sb, Bi):
Phosphabenzene (E = P) and arsabenzene (E = As) could thus be obtained as distillable liquids. Stibabenzene (E = Sb) was also obtained, but it is a labile substance that polymerizes quickly at room temperature. In contrast, bismabenzene (E = Bi) could not be isolated in substance, but could be detected spectroscopically and by targeted derivatization.
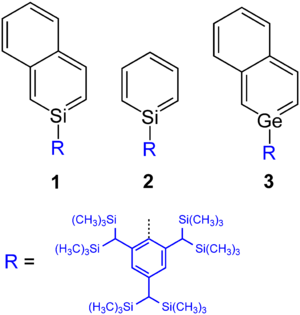
2-silanaphthalene 1 was produced by a Japanese group in 1997 as the first silabenzene derivative. The authors Norihiro Tokitoh and Renji Okazaki then reported on the synthesis of a thermally stable silabenzene 2 that is stabilized by a sterically demanding substituent.
The basic body germabenzene was considered theoretically, but not yet synthesized. In contrast, a 2-germanaphthalene-derived stable substance 3 has been produced.
Individual evidence
- ↑ a b C. Elschenbroich: Organometallics ( German ). Wiley-VCH, Weinheim 2006, ISBN 3-527-29390-6 , pp. 229-230.
- ↑ Christoph Elschenbroich: Organometallchemie . 6th edition. Teubner Verlag, Wiesbaden 2008, p. 218 ( limited preview in Google Book Search [accessed February 25, 2010]).
- ↑ a b Gottfried Märkl: Phosphabenzol and Arsabenzol. The higher element homologues of pyridine , Chemie in our time 16 (1982), pp. 139-148, doi : 10.1002 / ciuz.19820160503 .
- ^ A. Gossauer: Structure and Reactivity of Biomolecules , Wiley-VCH, Weinheim 2006, ISBN 3-906390-29-2 , p. 488.
- ^ A. Täufel, W. Ternes, L. Tunger, M. Zobel: Lebensmittel-Lexikon , 4th edition, p. 450, Behr Verlag, 2005, ISBN 3-89947-165-2 .
- ↑ GA Burdock (Ed.): Fenaroli's Handbook of Flavor Ingredients , Volume II, 3rd Edition, CRC Press, Boca Raton, 1995, ISBN 0-8493-2710-5 .
- ↑ A. Tschitschibabin in: J. Prakt. Chem. , 1924 , 107 , p. 122.
- ↑ a b A. J. Ashe: The Group 5 Heterobenzenes . In: Accounts of Chemical Research . 11, No. 4, 1978, pp. 153-157. doi : 10.1021 / ar50124a005 .
- ↑ Norihiro Tokitoh, Keiji Wakita, Renji Okazaki, Shigeru Nagase, Paul von Ragué Schleyer, Haijun Jiao: A Stable Neutral Silaaromatic Compound, 2- {2,4,6-Tris [bis (trimethylsilyl) methyl] phenyl} -2-silanaphthalene . In: Journal of the American Chemical Society . 119, 1997, pp. 6951-6952, doi : 10.1021 / ja9710924 .
- ↑ Keiji Wakita, Norihiro Tokitoh, Renji Okazaki, Shigeru Nagase, Paul von Ragué Schleyer, Haijun Jiao: Synthesis of Stable 2-Silanaphthalenes and Their Aromaticity. In: Journal of the American Chemical Society. 121, 1999, pp. 11336-11344, doi : 10.1021 / ja992024f .
- ↑ Keiji Wakita, Norihiro Tokitoh, Renji Okazaki, Nozomi Takagi, Shigeru Nagase: Crystal Structure of a Stable Silabenzene and Its Photochemical Valence Isomerization into the Corresponding Silabenzvalene. In: Journal of the American Chemical Society. 122, 2000, pp. 5648-5649, doi : 10.1021 / ja000309i .
- ↑ Ebrahimi, AA; Ghiasi, R .; Foroutan-Nejad, C .: Topological Characteristics of the Ring Critical Points and the Aromaticity of Groups IIIa to VIa Hetero-Benzenes . In: Journal of Molecular Structure: THEOCHEM . 941, No. 1-3, 2010, pp. 47-52. doi : 10.1016 / j.theochem.2009.10.038 .
- ↑ N. Nakata, N. Takeda, N. Tokitoh: Synthesis and Structure of a Kinetically Stabilized 2-Germanaphthalene: The First Stable Neutral Germaaromatic Compound . In: Organometallics . 20, No. 26, 2001, pp. 5507-5509. doi : 10.1021 / om010881y .