Hexamethyldisilane
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
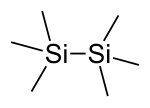
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Hexamethyldisilane | |||||||||||||||
| Molecular formula | Si 2 C 6 H 18 | |||||||||||||||
| Brief description |
colorless liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 146.38 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.715 g cm −3 (25 ° C) |
|||||||||||||||
| Melting point |
9-12 ° C |
|||||||||||||||
| boiling point |
112-114 ° C |
|||||||||||||||
| Refractive index |
1.422 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Hexamethyldisilane is a chemical compound from the group of silanes .
Extraction and presentation
Hexamethyldisilane can be obtained by reacting chlorotrimethylsilane with sodium in ethyl acetate at 200-230 ° C.
properties
Hexamethyldisilane is a colorless liquid.
use
Hexamethyldisilane is used as a silylating reagent for allyl acetates, aryl halides and diketones. It serves as the starting material for vapor deposition during silicon carbide growth. It is also the precursor of trimethylsilyl lithium , trimethylsilyl sodium and trimethylsilyl potassium by cleavage with lithium alkyls or alcoholates .
Individual evidence
- ↑ a b c d e f g h i j k data sheet hexamethyldisilane, 98% from Sigma-Aldrich , accessed on January 7, 2014 ( PDF ).
- ↑ Georg Brauer (Ed.), With the collaboration of Marianne Baudler a . a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume I, Ferdinand Enke, Stuttgart 1975, ISBN 3-432-02328-6 , p. 706.
- ↑ Data sheet hexamethyldisilane, 98 +% from AlfaAesar, accessed on January 7, 2014 ( PDF )(JavaScript required) .



