Large piston water beetle
| Large piston water beetle | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|

Large piston water beetle ( Hydrous piceus ) |
||||||||||||
| Systematics | ||||||||||||
|
||||||||||||
| Scientific name | ||||||||||||
| Hydrous piceus | ||||||||||||
| Linnaeus , 1775 |
The Big Piston water beetles ( Hydrophilus piceus , Syn. : Hydrous piceus ), whose name is on its size and bulbous thickened probe is due is, with a length of up to five centimeters, the largest water beetles so early in Europe and was also a giant water bugs and Big Fish beetle called . It shows some very interesting adaptations to aquatic life in breathing and brood care and is under nature protection because of its increasing endangerment .
Naming
According to EuroFauna, the large piston water beetle has the scientific name Hydrophilus piceus , but in German-language literature it is largely carried under the name Hydrous piceus . The generic name " Hydrous Linnaeus 1775" is used by EuroFauna as a generic synonym for " Hydrophilus Geoffroy 1762". H. piceus belongs to the family of Hydrophilidae what with Wasserfreunde be translated would have, the family is now on but usually German water beetles named (s. Str. = Strict sense, in the strict sense). Since the term water beetle (s. L. = Sensu lato, in a broader sense) is also used for the ecological group of all beetles living in the water, which, in addition to the species of the Hydrophilidae family, also includes the families of the real swimming beetles , it would actually make more sense to call the Hydrophilidae water lovers to call. This is also supported by the fact that several species of the Hydrophilidae do not even live in the water.
For further confusion, the species name piceus (Latin) should be translated as pitch black, but the very similar beetle with the scientific name Hydrophilus aterrimus is referred to as the black piston water beetle .
construction
The piston water beetle is inconspicuously colored in a matt black with a brown-olive shimmer. The sensor lobe is brownish, the rest of the sensor and the buttons are rust-red to amber-colored (Fig. 1 and 3). In Hydrous , the head, pronotum and elytra are close together and are so uniformly rounded that the body outline forms a fairly closed oval when viewed from above. The upper side is clearly arched, with the highest point in the front third of the wing cover, the lower side, however, is relatively flat, the chest and abdomen are keeled. In H. piceus the keel of the abdomen is clear and not rounded as in the closely related H. aterrimus . This creates a streamlined shape that is not, however, suitable for fast swimming.
Each wing cover has eight inconspicuous rows of points, in the intervals between there are irregular rows of points . In contrast to H. pistaceus , the wing cover has a sharp tooth at the tip of the seam (Fig. 4). In H. piceus the thoracic keel is deeply furrowed at the front and drawn out into a free-standing pointed thorn at the rear, which protrudes far beyond the rear hips (Fig. 10). In Fig. 5 the spike pointing down to the right can be clearly seen. The name sting is popular for this thorn , because if you want to encircle the beetle with your hand, it can sting sensitively with a jerky backward movement with the thorn. In the past, the beetle was also called carp piercer , because it was believed that it would pierce carp with this thorn. Between the abdomen and the top of the wing there are structures that rub against each other and produce sounds. The male uses it to produce sounds that are answered by the female.
The hydrophilids belong to the Palpicornia family series, which are characterized by their long jaws (maxillary palps). In Fig. 1 you can see the feeler protruding horizontally on the right in front of the eye, underneath the downward curved long jaw probe and at the very bottom just a little below the head the tips of the lip probe. The jaw probes are four-limbed, the first limb is small, the two following are very long and thin, the end limb is blunt and only half as long as the third limb. They are longer than the feelers and also take on their function as tactile organs. The antennae (Fig. 3) are nine-membered, the first member long shaft-shaped, the second conical member together with the following three members form a hostage. The last four links form a loose club, which can be folded down between its first and second link by a hinge and the last three links are hollowed out in the shape of a spoon, so that the hollows together on the side of the body form a groove limited by hairs. The lip buttons are three-part, the first part very small and round, the second cylindrical and long, the last short and blunt. The jaw probe and usually the lip probe protrude clearly from the outline of the body, while the feelers are worn so close to the body that they are easily overlooked. If you look at it superficially, you can mistake the jaw buttons for the feelers. The upper jaws are curved at the tip, deeply split and serrated on the inner edge.
The hydrophilids belong to the pentamers, accordingly the tarsi of the water beetle are five-limbed on all legs . The foreleg shows an abnormality only in the males (Fig. 2). With them, the claw member is broadly widened in the shape of a hatchet, the two claws of the pair of claws are not symmetrical, but the claw approaching the extension is significantly wider and specialized in performing the function of a forceps in connection with the extension.
The middle and rear legs are designed as swimming legs with flattened tarsal limbs (Figs. 6 and 7). On the inside the tarsus is widened by a series of elastic hairs and on the outside by a series of small teeth. Depending on their position, the tarsi can offer greater or lesser resistance to the water. On the side opposite the hair, the tarsal links overlap. When the legs are thrown back vigorously, which propel the beetle forward, they increase stability and allow the inward curvature to be slightly reduced, thereby increasing the area effective for propulsion. In addition, in the middle and rear tarsi, a slight rotation around the tarsal axis is possible between the first and second tarsal segments. During the stroke backwards, the area of the tarsus perpendicular to the direction of stroke can be increased; when the legs are pulled back against the body, the tarsus is rotated so that its area perpendicular to the direction of movement becomes small. Various other adjustments support the possibility of effective swimming. The hips of the swimming legs are sunk into the sternum and their mobility is therefore severely restricted. This makes the swimming movement more stable. The joint between the hip and hip ring is shaped in such a way that during the entire leg kick it only allows the thigh to move parallel to the surface of the abdomen. The thigh is flattened in such a way that it offers a large surface area while stretching, but offers as little water resistance as possible when you bend afterwards. Nevertheless, the piston water beetle is a poor swimmer in comparison with the yellow fire beetle . It also has small claws on its swimming legs and almost only swims by paddling, i.e. using its hind legs as an alternative. On the other hand, its mobility is greater when climbing between aquatic plants and when walking on the ground.
breathing
The piston water beetle comes with its front end to the surface of the water to breathe. He holds his head from below to the water level and leans slightly to one side. Then he takes the feeler closer to the surface of the water from the air-filled pit on the underside of the pronotum. A pointed extension of the first link of the antenna lobe breaks through the skin of the water surface caused by the water surface tension from below. Then the sensor is bent and pushed out over the surface of the water so that the tip of the sensor remains below the surface of the water and lies against the furrow of the head. The head furrow is a groove consisting of two hairlines that runs vertically. It is supplemented by the overlying cavities of the three antennae end links (clearly visible in Fig. 3) to form a snorkel that extends from the bent antennae joint over the water to under the pronotum. The feeler lobe, which is covered with the finest hairs, performs vibrating movements for air transport. This activity is carried out alternately on the left and right, so that the animal rocks back and forth. However, Horion writes that when breathing the whole body makes trembling movements. A picture of the bent sensor during breathing can be found in various places on the Internet.
On the underside of the body, the beetles have dense golden-yellow hairs (pubescence), which can be seen in Fig. 10 by the brown coloration between the second and third pair of legs and along the edges of the wing cover, and in Fig. 5 to the left of the thorn above the thoracic keel. The air supply is transported in this hair. This air cushion on the underside of the body is held by the keel and the protruding edges of the wing panels and extends to the stigmas of the first abdominal segments. The layer of air is called plastron, which was originally used to describe the breast leather of armor. The oxygen dissolved in the water can diffuse out of the water into the plastron. There is also an air-filled space under the wing covers that is connected to the plastron. However, it is relatively small and insignificant. The part of the underside covered by the plastron appears shiny and silvery in the water due to the air bubbles adhering to the hair. There are reports that the animal has to take a breath every five minutes, but that certainly depends on the oxygen content of the water. This can fluctuate extremely, especially in the areas covered with aquatic plants.
Brood care
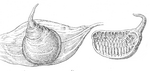
The female of the piston water beetle shows an unusual form of brood care . To accommodate the eggs, she builds what is known as a boat (Fig. 8). It is about two centimeters long, flat at the bottom, arched at the top and provided with a "chimney". Of the numerous detailed descriptions of the manufacture of this shuttle, the "old Brehm" should be followed mainly because of the linguistic originality, the original passages appear in italics.
The female looks for a leaf floating on the water, to which it holds on from below with its front legs. Instead of the single sheet and some may duckweed occur. The attachment glands of the genital organs located in the end of the abdomen form a spider secretion that emerges from the vagina. At the end of the abdomen there are two thin extensions, the spinning rods, which can guide the spinning thread through its movement. The four whitish threads flowing from the abdomen are woven into a web spanning the entire abdomen of the animal by moving the tip of the body back and forth and attached to the underside of the leaf. When this is done, the beetle turns around, taking the web on its back (Fig. 12). The beetle is now holding onto the leaf with its hind legs. Its ventral surface to Form benutzend made it a second plate which is fastened together with the first on the sides. Finally his abdomen is stuck in a sack that is open to the front. He fills it from behind with rows of eggs, and moves out of it as they increase, until at last the bag is filled and the tip of the abdomen slips out. Now he grips the edges with his back legs and spins thread by thread until the opening becomes narrower and narrower and has a somewhat bulky hem. Then he pulls threads up and down across and completes the end like a lid. A tip in the shape of a slightly curved croissant is placed on this lid (Fig. 8). The work is completed in four to five hours. The upper part of this woven bag is only filled with loose weave, on the underside there are around 50 eggs. That is why the shuttle straightens up again despite the "chimney" if it is accidentally knocked over. The chimney should ensure the air supply. In addition to this ingenious brood care, in contrast to other types of the family, Hydrous does not provide brood care . The female no longer cares about the fate of the offspring.
Larva and pupa
The blackish larva (Fig. 9 and Fig. 13) also lives in the water. To breathe it brings the abdomen to the surface of the water and uses a stigma at the end of the abdomen. It resembles a long, soft worm with a chitinized head with two strong and pointed jaws. On the underside of the head there is a field with single eyes on both sides. Their antennae are long and thin, but, as in adults, shorter than the maxillary palps. They do not play a role in breathing. The head can be moved in all directions and is normally held rotated 90 degrees upwards to the horizontally moving body axis. Due to the great mobility of the head, the larva can grab water snails swimming on the water surface without having to change the horizontal position of the body while swimming. The head can even be bent back so extreme that the larva can use its back as a support when consuming the prey. The legs are very simply built and in no way specialized as swimming legs. Nevertheless, due to their light specific weight and the mobility of the body, the larvae swim well, very fish-like in the water after predation. At the end of the body there are two fleshy appendages with which the larvae can hang upside down on the surface of the water. The larva has the habit of pretending to be dead and hanging down like an empty hide on either side of the finger that holds it. If this ruse does not want to help, it clouds its immediate surroundings with a black, stinking juice that oozes from the anus and thus protects itself more often from persecution.
The doll (Fig. 11) is a free doll, that is, the limbs and body are not surrounded by a common shell. Legs, mouthparts, eyes, antennae and the thorn are already clearly visible. The pupa has bifurcated growths on both sides of the head near the pronotum. These “horns” of the doll are probably used to smooth the doll's cavity.
biology
In the boat in which the female laid the eggs (Fig. 8), the larvae hatch after 16 to 18 days. However, they will probably remain in the cocoon until the first molt. The larvae crawl around on the bottom of shallow waters and mainly hunt water snails, whose shells large larvae can partially break open, otherwise they eat their way deep into the shells. But as predators they are not picky about their prey. Usually they have to be content with insect larvae and water snails, of which they mainly hunt the poppy snails of the genus Planorbis and the mud snails of the genus Lymnaea. Since the sharp jaws are not provided with a channel through which digestive juices can be injected into the prey, as is the case with the Dytiscidae, the larvae vomit gastric juices onto the prey for pre-digestion, as is also known with ground beetles. Since the water would significantly reduce the effectiveness of the digestive juices, the larvae lift the prey out of the water when they eat. The food pulp liquefied by the pre-digestion is then absorbed. The larva goes through three larval stages until it pupates after four to six weeks in midsummer. To do this, they often cover several meters on land until they find a suitable subsoil that is still damp, but not threatened by the water and not compacted. There they build a cave in the ground in which they pupate. In the same autumn the beetles hatch and wait in the pupation cavity for the chitin to harden and color. Then they break open the cave, which often catches the eye as a globe that is smooth on the inside and rough on the outside. They go into the water and feed on rotting aquatic plants, mainly crawling around in the bank area. It is discussed whether the beetle also accepts animal prey. There are photos showing the beetle on the carrion of a small fish. The main food, however, is undoubtedly of a vegetable nature. When the beetle moves by swimming, it usually uses its hind legs alternatively, but it can also use them synchronously when swimming faster. Since the beetles prefer shallow waters in summer, they have to face the risk of the waters drying out. They undertake long search flights at night. He is attracted to light. The beetle can live up to three years. It hibernates in the water and needs water that is deep enough not to freeze to the bottom. Mating takes place in spring.
Occurrence
Hydrous piceus is distributed in the Palearctic . In Europe, the northern limit of distribution is in southern England and Denmark. It is also found in North Africa, the Middle East and as far as Siberia.
It prefers standing water that has at least areas rich in vegetation, but is not too eutrophic. The type of subsoil is irrelevant. Because of the different demands at different times of the year, the beetle is most often found in water complexes, for example in lake plateaus or in ditch systems for the irrigation of agricultural areas, especially rice fields. The distribution area shows that warmer waters are preferred. Apparently it does not occur in salt or brackish water. Because of its good flight ability, one cannot conclude from a find in a body of water that the beetle is native there. Occasional finds in brackish water would then be interpreted as missed flights.
protection
The large piston water beetle is also called pitch black piston water beetle and large black piston water beetle in the Red Lists. In the Red Lists of the states of Bavaria , Saxony and Saxony-Anhalt the species is listed under the category "endangered". In Brandenburg it is considered "endangered", in Schleswig-Holstein as "threatened with extinction" and in Thuringia as "extinct or lost".
The piston water beetle is a good example of the fact that it is of little use to put a beetle under protection and thus forbid its collection or use in any form. The life requirements of the beetle must be examined more closely and appropriate accompanying protective measures taken. Hydrous piceus has been under nature protection in Germany since 1936, is included in numerous Red Lists and has been included in the Federal Species Protection Ordinance since 1980. However, its population is steadily declining, although its main natural predators, the waterfowl, are also becoming rarer. The main causes for this decline were found to be pollution, islanding and shading of the residential waters. The pollution caused by the discharge of sewage and the washing in of fertilizers leads to stronger plant growth and, when they break down, to a lack of oxygen, which in turn causes the decline of snails, which are the main food of the beetle larvae. Feeding that is supposed to compensate for this deficiency does not make much sense since, for example, releasing small fish, intended as food for the large larvae, eats smaller larvae and acts as food competitors with regard to food. The easiest way to counteract shading. Trees standing on the bank can be felled. Unfortunately, this usually goes hand in hand with a development for nature-based tourism, such as the creation of cycling and hiking trails. This in turn leads to the consolidation and drying out of the bank region, which means that there is no longer any subsurface available for the pupation cavity. The most difficult thing to do is to counteract isolation. The beetle needs shallower and deeper bodies of water, which may require complex construction measures to create artificial, near-natural lakes.
The danger posed by road traffic should not be underestimated, as the clumsy animals attracted by the light are helpless.
Individual evidence
- ↑ Synonyms for hydrophilus
- ↑ a b c d e Kurt Lampert: Pictures from the life of the beetle. Strecker and Schröder, Stuttgart 1909
- ↑ a b James Duncan: Beetles, British and Foreign David Bogue, London 1850
- ↑ a b Bernhard Klausnitzer: Wonderful world of the beetles. Herder, Freiburg 1982, ISBN 3-451-19630-1
- ↑ G. Jäger (Ed.): CG Calwer 's Käferbuch . K. Thienemanns, Stuttgart 1876, 3rd edition
- ↑ Swimming leg (PDF, 36 kB)
- ↑ a b Rororo Wildlife. The Urania animal kingdom in 18 volumes. Volume 2. Urania, Leipzig-Berlin-Jena 1971, ISBN 3-499-28011-6
- ^ Adolf Horion: Käferkunde for nature lovers. Vittorio Klostermann, Frankfurt am Main 1949
- ↑ Image of the sensor when breathing ( Memento from October 8, 2007 in the Internet Archive )
- ^ A b Svatopluk Bílý: Coléoptères, adaptation française. Gründ, Paris 1990, ISBN 2-7000-1824-9
- ↑ Frequency of breathing
- ↑ Picture of the bent probe ( memento of October 8, 2007 in the Internet Archive )
- ↑ Wolfgang Engelhard: What lives in pools, brooks and ponds? Kosmos, Stuttgart 1955
- ↑ a b c Brehm's animal life. Volume 5. Schlüter Vertriebsgesellschaft Leipzig 1930
- ↑ Henri Bertrand: Larves et Nymphes of Coléoptères aqatiques du Globe. Imprimerie Paillard, Paris 1972
- ↑ Natural history of the animal kingdom. JF Schreiber, Esslingen 1886
- ↑ a b Endangerment and Protection ( Memento of October 7, 2007 in the Internet Archive ) (PDF, 36 kB)
- ↑ Red Lists of BioNetworkX
literature
- Heinz Joy, Karl Wilhelm Harde, Gustav Adolf Lohse: The beetles of Central Europe . tape 3 . Adephaga 2 - Staphylinoidea 1. Goecke & Evers, Krefeld 1971, ISBN 3-87263-015-6 .
- Bernhard Klausnitzer : Beetles in and around the water. 2nd, revised edition. The new Brehm library, Volume 567. Westarp Sciences and Spectrum, Academic Publishing House, Magdeburg, Heidelberg, Berlin and Oxford 1996, ISBN 3-89432-478-3