Indium (II) selenide
| Crystal structure | |||||||
|---|---|---|---|---|---|---|---|
|
|||||||
|
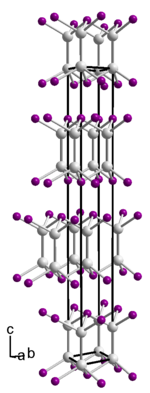
__ In 2+ __ Se 2− Crystal structure of the γ form of indium (II) selenide |
|||||||
| General | |||||||
| Surname | Indium (II) selenide | ||||||
| Ratio formula | InSe | ||||||
| Brief description |
black crystals |
||||||
| External identifiers / databases | |||||||
|
|||||||
| properties | |||||||
| Molar mass | 193.78 g mol −1 | ||||||
| Physical state |
firmly |
||||||
| Melting point |
660 ° C |
||||||
| safety instructions | |||||||
|
|||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||
Indium (II) selenide (InSe) is a chemical compound of indium and selenium , a so-called III-VI semiconductor . In 2 Se 3 , In 4 Se 3 and In 6 Se 7 are to be distinguished from InSe .
Extraction and presentation
The substance can be obtained by reacting indium with selenium.
properties
Indium (II) selenide is a black, matt, greasy, glossy, easily friable solid. It's polymorphic . β-InSe crystallizes in the space group P 6 3 / mmc (space group no.194 ) , ε-InSe in the space group P 6 m 2 (no. 187) and γ-InSe in the space group R 3 m ( no.160 ) . A polymorph in the space group P 6 3 22 (No. 182) is also described. The dominant phase is the γ form. The lattice parameters of the γ-form are a = 4.002 Å and c = 24.95 Å. The structure consists of In 2 4+ cations, which are bound to neighboring cations by bridging selenium atoms, so that a layered structure results. The layered structure results in two band transitions , an indirect band transition with a band gap energy of approx. 1.2 eV and a direct band transition with approx. 2.4 eV.
literature
- R. Clasen, O. Madelung: Semiconductors. Physics of Non-tetrahedrally Bonded Binary Compounds II . In Landolt-Börnstein numerical data and functional relationships in science and technology. New series. Group III, Crystal and solid state physics . Volume 17, Springer, ISBN 3-540-12160-9 ( limited preview in Google book search)
Individual evidence
- ↑ a b c Entry on indium selenide. In: Römpp Online . Georg Thieme Verlag, accessed on July 15, 2014.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ a b Georg Brauer (Ed.), With the collaboration of Marianne Baudler u a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume I, Ferdinand Enke, Stuttgart 1975, ISBN 3-432-02328-6 , p. 872.
- ↑ T. Ikari, S. Shigetomi, K. Hashimoto: Crystal Structure and Raman Spectra of InSe. In: Basic Solid State Physics , 111 (2), 1982, pp. 477-481, doi: 10.1002 / pssb.2221110208 .
- ↑ S. Inoue, T. Yoshida, T. Morita: Crystal Structure of InSe Grown from Melt and Its Structure Transition. In: Japanese Journal of Applied Physics , 21 (2), 1982, pp. 242-248.
- ↑ JA Hollingsworth, DM Poojary, A. Clearfield, WE Buhro: Catalyzed Growth of a Metastable InS Crystal Structure as Colloidal Crystals. In: Journal of the American Chemical Society , 122, 2000, pp. 3562-3563, doi: 10.1021 / ja000106u .
- ↑ J. Rigoult, A. Rimsky: Refinement of the 3R γ-indium Monoselenide StructureType. In: Acta Crystallographica , B36, 1980, pp. 916-918, doi: 10.1107 / S0567740880004840 .
- ↑ John Vincent McCanny , RB Murray: The band structures of gallium and indium selenide. In: Journal of Physics C: Solid State Physics. 1977, No. 10, 1977, pp. 1211-1222, doi: 10.1088 / 0022-3719 / 10/8/022
