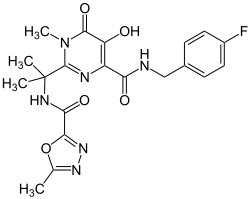
Raltegravir
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Non-proprietary name | Raltegravir | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 20 H 21 FN 6 O 5 | ||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| Drug information | |||||||||||||||||||
| ATC code | |||||||||||||||||||
| Drug class | |||||||||||||||||||
| Mechanism of action | |||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 444.41 g · mol -1 | ||||||||||||||||||
| solubility |
water soluble |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Raltegravir (trade name: Isentress ; manufacturer: MSD Sharp & Dohme ) is a drug from the group of integrase inhibitors that is used to treat HIV- infected patients.
Isentress is the first approved drug from the group of integrase inhibitors. Raltegravir is said to be better tolerated compared to other drugs used in HAART ; It is also hoped that it will be effective in patients with multi-resistant viruses.
history
Isentress was approved in the US on October 12, 2007 and in the European Union on December 20, 2007. Raltegravir is the first representative of this new class of integrase inhibitors to be launched on the market. In the EU, only a conditional approval was initially granted, which obliged the manufacturer to submit further study results from two phase III studies in the course of the year, which are included in the assessment of the risk-benefit ratio. On July 14, 2009, the “subject to conditions” approval was revoked. The unrestricted marketing authorization is valid for five years and can then be extended. The Federal Patent Court has on 31 August 2016 way of interlocutory a compulsory license granted to the drug, which the Federal Court has confirmed in its judgment of 11 July 2017th
Clinical information
Application areas (indications)
Raltegravir is approved for the combination therapy of HIV- infected adults who have proven HIV-1 replication despite antiretroviral therapy.
Contraindications (contraindications)
Hypersensitivity to the active ingredient.
Side effects
The most common (> 10%) adverse events during the clinical trials were diarrhea , nausea, headache and fever. The rate of discontinuation due to adverse events in raltegravir-treated patients was 2 percent compared to 1.4 percent for placebo. Experience from long-term treatment is not yet available.
pharmacology
Pharmacodynamics
Raltegravir works as an integrase inhibitor. The retroviral integrase is a key enzyme that the HIV virus needs for the integration of its genome into the chromosomes of the host cell . Raltegravir inhibits an essential catalytic step of this enzyme, strand transfer, thereby preventing the integration of the viral nucleic acid and consequently reducing virus replication. A number of other enzymes, including retroviral reverse transcriptase , were tested to see whether they would be inhibited by raltegravir. This could not be observed; it is considered certain that the antiviral activity can be attributed exclusively to the inhibition of integrase. The body's own enzymes are not affected.
Pharmacokinetics
Raltegravir is rapidly absorbed ; meal intake is little affected. The drug is metabolized by glucuronidation . The terminal half-life is given as about nine hours.
toxicology
There is no specific information on patient overdose. The toxicity determination was carried out on mice, rats and dogs. A single oral administration of raltegravir was well tolerated in the mouse ( LD50 > 2000 mg / kg); for intravenous administration, the highest tolerated dose was 100 mg / kg / day. After repeated oral administration, 50 mg / kg / day was well tolerated. In similar studies in rats, a NOAEL of 120 mg / kg / day was determined. No damage was found in dogs after oral administration of> 360 mg / kg / day for one year. In studies of genotoxicity and reproductive toxicity, the substance was normal. Carcinogenicity studies are ongoing.
Individual evidence
- ↑ a b c d e f g h Public assessment report (EPAR) of the European Medicines Agency (EMA) on: Isentress .
- ↑ Registration dossier on N - [(4-fluorophenyl) methyl] -1,6-dihydro-5-hydroxy-1-methyl-2- [1-methyl-1 - [[(5-methyl-1,3,4- oxadiazol-2-yl) carbonyl] amino] ethyl] -6-oxo-4-pyrimidinecarboxamide ( GHS section ) from the European Chemicals Agency (ECHA), accessed on July 11, 2020.
- ↑ Press release of the Federal Court of Justice of July 11, 2017 No. 111/17, accessed on July 11, 2017