Potassium hexafluoroantimonate (V)
| Crystal structure | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| __ K + __ Sb 5+ __ F - | |||||||||||||||||||
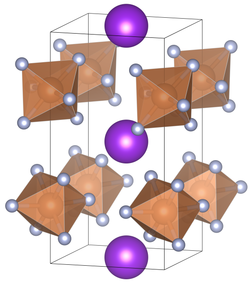
| Crystal system |
tetragonal |
||||||||||||||||||
| Space group |
P 4 2 m (No. 111) |
||||||||||||||||||
| Lattice parameters |
a = 5.16 Å, c = 10.07 Å |
||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Potassium hexafluoroantimonate (V) | ||||||||||||||||||
| Ratio formula | K [SbF 6 ] | ||||||||||||||||||
| Brief description |
odorless, white powder |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 274.85 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
846 ° C |
||||||||||||||||||
| boiling point |
1505 ° C |
||||||||||||||||||
| solubility |
soluble in water |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Potassium hexafluoroantimonate , K [SbF 6 ] is an inorganic compound between the alkali metal potassium and the superacid hexafluoroantimonic acid .
Extraction and presentation
Potassium hexafluoroantimonate can be prepared by reacting potassium pyroantimonate K 2 H 2 Sb 2 O 7 with hydrogen fluoride or by treating the product of the reaction of a mixture of solid antimony (III) oxide and potassium hydroxide with hydrogen peroxide solution (30%) with hydrochloric acid (48%) become. The compound can also be synthesized by treating an equimolar mixture of antimony (V) chloride and potassium chloride (or potassium fluoride ) with an excess of anhydrous hydrogen fluoride at high temperatures and pressures.
properties
Potassium hexafluoroantimonate is an odorless, white powder. It can be dissolved in water. Potassium hexafluoroantimonate crystallizes depending on the temperature in a tetragonal (coordination number of the A-position = 8) with the space group P 4 2 m (space group no. 111) and a cubic (coordination number of the A-position = 6) variant with the space group Ia 3 (room group no.206) . The compound undergoes at 16 ° C a transition from a cubic to tetragonal crystal structure . Another crystal structure is known at high pressure.
use
Potassium hexafluoroantimonate is used as a pharmaceutical intermediate.
safety instructions
The compound is toxic if swallowed or inhaled .
The salt can cause violent reactions with strong acids and oxidizing agents . When the substance is burned, hydrogen fluoride , potassium oxide and antimony oxides can be produced.
Individual evidence
- ^ A b G. J. Kruger, CWFT Pistorius, AM Heyns: Potassium hexafluoroantimonate (I) . In: Acta Crystallographica Section B . B32, 1976, p. 2916-2918 , doi : 10.1107 / S0567740876009230 ( Open Access ).
- ↑ a b Data sheet Potassium hexafluoroantimonate from ApolloScientific, accessed on February 16, 2019 ( PDF ).
- ↑ a b c d data sheet Potassium hexafluoroantimonate (V) from Sigma-Aldrich , accessed on February 16, 2019 ( PDF ).
- ↑ a b c d data sheet Potassium hexafluoroantimonate (V) from Santa Cruz Biotechnology, accessed on February 16, 2019 ( PDF ).
- ↑ a b c d e f data sheet Potassium hexafluoroantimonate from AlfaAesar, accessed on February 16, 2019 ( PDF )(JavaScript required) .
- ↑ a b Jane E. Macintyre: Dictionary of Inorganic Compounds . CRC Press, 1992, ISBN 978-0-412-30120-9 , pp. 3217 ( limited preview in Google Book search).
- ↑ Caroline Röhr, Rüdiger Kniep: The crystal structures of Li [PF 6 ] and Li [AsF 6 ]: On the crystal chemistry of compounds A [EVF 6 ]. In: Journal of Nature Research B . 49, 1994, pp. 650-654 ( PDF , free full text).
- ↑ Hans Bode, Ernst Voss: The crystal structure of potassium hexafluoroantimonate (V) . In: Journal of Inorganic and General Chemistry . tape 264 , no. 2-4 , March 1951, pp. 144-150 , doi : 10.1002 / zaac.19512640208 .
- ^ Anton M. Heyns, Carl W. F. T. Pistorius: Polymorphism, high-pressure phase diagram and vibrational spectra of KSbF 6 . In: Spectrochimica Acta Part A . tape 32 , no. 3 , 1976, p. 535-545 , doi : 10.1016 / 0584-8539 (76) 80114-6 .

